Staphylococcus aureus (S. aureus) is considered one of the most widespread infectious bacteria. It is found in the environment as well as being part of the human skin and nasal microbiota. Normally, S. aureus is harmless on healthy skin, but once it enters the blood or internal tissues, diverse infections occur including pneumonia, infection of surgical site and nosocomial bacteremia. Systemic S. aureus infection depends on the bacteria breaking through the epithelial protective layer. The incidence rate of this serious medical condition is between 20 and 50 cases/100,000 per year, with fatality rate ranging from 10% to 30%. Moreover, S. aureus forms biofilms that are associated with medical device infections such as prosthetic joints and endocarditis. The prevalence of antibiotic resistance S. aureus isolates, methicillin-resistant S. aureus (MRSA), is posing a serious problem for combating infectious diseases caused by this pathogen.
- metallophores
- virulence
- Staphylococcus aureus
1. Siderophores
2. Additional Metal Acquiring Systems
2.1. Metallophore Staphylopine
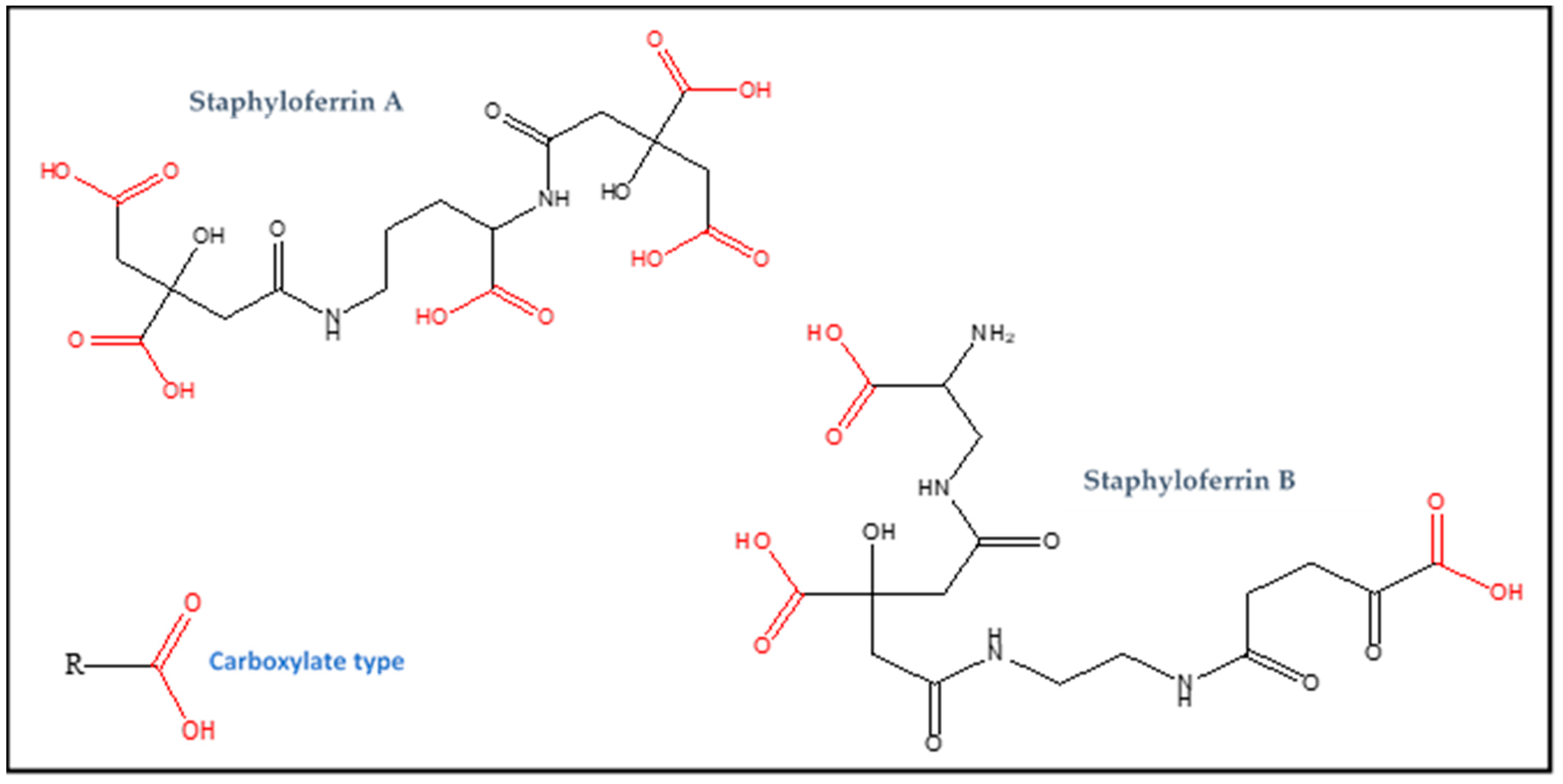
2.2. Staphylopine Synthesis
| Name | Type (Functional Group) |
Molecular Formula | Molecular Weight (Da) | Specific for |
|---|---|---|---|---|
| Staphyloferrin A | Carboxylate (RCOOH) | C17H24N2O14 | 480 | Iron |
| Staphyloferrin B | Carboxylate (RCOOH) | C16H24N4O11 | 448 | Iron |
| Staphylobactin | Hydroxamate (R-CO-NH-OH) |
unknown | 822 | Iron |
| Aureochelin | hydroxamate and catechol (R-CO-NH-OH and C6H4(OH)2) |
unknown | 577 | Iron |
| Staphylopine | opine (amine and carboxylic acid) |
C13H19N4O6− | 327 | broad-spectrum metallophore (nickel, zinc, cobalt, iron and copper) |
| Name | Operon | Metal Uptake Regulator | Membrane Transporter |
|---|---|---|---|
| Staphyloferrin A | sfaABCD | Fur | HtsABC |
| Staphyloferrin B | sbn (sbnABCDEFGHI) | Fur | SirABC |
| Staphylobactin | sbn | Fur | SirABC |
| Staphylopine | cnt (cntKLMABCDFE) | Fur and Zur | CntB and CntC |
2.3. Staphylopine as Zincophore
2.4. Important Features of Cnt-Staphylopine System
This entry is adapted from the peer-reviewed paper 10.3390/biology11101525
References
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202.
- Liu, L.; Wang, W.; Wu, S.; Gao, H. Recent Advances in the Siderophore Biology of Shewanella. Front. Microbiol. 2022, 13, 823758.
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82, 969–974.
- Emerson, D.; Roden, E.; Twining, B.S. The microbial ferrous wheel: Iron cycling in terrestrial, freshwater, and marine environments. Front. Microbiol. 2012, 3, 383.
- Kramer, J.; Özkaya, O.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163.
- Sebulsky, M.T.; Hohnstein, D.; Hunter, M.D.; Heinrichs, D.E. Identification and characterization of a membrane permease ivolved in iron-hydroxamate transport in Staphylococcus aureus. J. Bacteriol. 2000, 182, 4394–4400.
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451.
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519.
- Challis, G.L. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. Chem. Bio. Chem. 2005, 6, 601–661.
- Carroll, C.S.; Moore, M.M. Ironing out siderophore biosynthesis: A review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 356–381.
- Crosa, J.H.; Walsh, C.T. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249.
- Lee, J.Y.; Janes, B.K.; Passalacqua, K.D.; Pfleger, B.F.; Bergman, N.H.; Liu, H.; Håkansson, K.; Somu, R.V.; Aldrich, C.C.; Cendrowski, S.; et al. Biosynthetic analysis of the petrobactin siderophore pathway from Bacillus anthracis. J. Bacteriol. 2007, 189, 1698–1710.
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003, 27, 215–237.
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630.
- Faraldo-Gómez, J.D.; Sansom, M.S.P. Acquisition of siderophores in gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 2003, 4, 105–116.
- Schalk, I.J.; Guillon, L. Fate of ferrisiderophores after import across bacterial outer membranes: Different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 2013, 44, 1267–1277.
- Ganne, G.; Brillet, K.; Basta, B.; Roche, B.; Hoegy, F.; Gasser, V.; Schalk, I.J. Iron release from the siderophore pyoverdine in Pseudomonas aeruginosa involves three new actors: FpvC, FpvG, and FpvH. ACS Chem. Biol. 2017, 12, 1056–1065.
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta 2008, 1778, 1781–1804.
- Imperi, F.; Tiburzi, F.; Visca, P. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2009, 106, 20440–20445.
- Lin, H.; Fischbach, M.A.; Liu, D.R.; Walsh, C.T. In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J. Am. Chem. Soc. 2005, 127, 11075–11084.
- Remy, L.; Carrière, M.; Derré-Bobillot, A.; Martini, C.; Sanguinetti, M.; Borezée-Durant, E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol. Microbiol. 2013, 87, 730–743.
- Lebrette, H.; Brochier-Armanet, C.; Zambelli, B.; de Reuse, H.; Borezée-Durant, E.; Ciurli, S.; Cavazza, C. Promiscuous nickel import in human pathogens: Structure, thermodynamics, and evolution of extracytoplasmic nickel-binding proteins. Structure 2014, 22, 1421–1432.
- Eitinger, T.; Suhr, J.; Moore, L.; Smith, J.A. Secondary transporters for nickel and cobalt ions: Theme and variations. Biometals 2005, 18, 399–405.
- Lebrette, H.; Borezée-Durant, E.; Martin, L.; Richaud, P.; Erba, E.B.; Cavazza, C. Novel insights into nickel import in Staphylococcus aureus: The positive role of free histidine and structural characterization of a new thiazolidine- type nickel chelator. Metallomics 2015, 7, 613–621.
- Ghssein, G.; Brutesco, C.; Ouerdane, L.; Fojcik, C.; Izaute, A.; Wang, S.; Hajjar, C.; Lobinski, R.; Lemaire, D.; Richaud, P.; et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 2016, 352, 1105–1109.
- Hiron, A.; Posteraro, B.; Carrière, M.; Remy, L.; Delporte, C.; La Sorda, M.; Sanguinetti, M.; Juillard, V.; Borezée-Durant, E. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol. Microbiol. 2010, 77, 1246–1260.
- Grim, K.P.; San Francisco, B.; Radin, J.N.; Brazel, E.B.; Kelliher, J.L.; Párraga Solórzano, P.K.; Kim, P.C.; McDevitt, C.A.; Kehl-Fie, T.E. The Metallophore Staphylopine Enables Staphylococcus aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 2017, 8, e01281-17.
- Ghssein, G.; Matar, S.F. Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation 2018, 6, 56.
- Hiron, A.; Borezee-Durant, E.; Piard, J.C.; Juillard, V. Only one of four oligopeptide transport systems mediates nitrogen nutrition in Staphylococcus aureus. J. Bacteriol. 2007, 189, 5119–5129.
- Ding, Y.; Fu, Y.; Lee, J.C.; Hooper, D.C. Staphylococcus aureus NorD, a putative efflux pump coregulated with the Opp1 oligopeptide permease, contributes selectively to fitness in vivo. J. Bacteriol. 2012, 194, 6586–6593.
- Song, L.; Zhang, Y.; Chen, W.; Gu, T.; Zhang, S.Y.; Ji, Q. Mechanistic insights into staphylopine-mediated metal acquisition. Proc. Natl. Acad. Sci. USA 2018, 115, 3942–3947.
- Lindsay, J.A.; Foster, S.J. zur: A Zn(21)-responsive regulatory element of Staphylococcus aureus. Microbiology 2001, 147, 1259–1266.
- Fojcik, C.; Arnoux, P.; Ouerdane, L.; Aigle, M.; Alfonsi, L.; Borezée-Durant, E. Independent and Cooperative Regulation of Staphylopine Biosynthesis and Trafficking by Fur and Zur. Mol. Microbiol. 2018, 108, 159–177.
- Hajjar, C.; Fanelli, R.; Laffont, C.; Brutesco, C.; Cullia, G.; Tribout, M.; Nurizzo, D.; Borezée-Durant, E.; Voulhoux, R.; Pignol, D.; et al. Control by Metals of Staphylopine Dehydrogenase Activity during Metallophore Biosynthesis. Am. Chem. Soc. 2019, 141, 5555–5562.
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth tissue abscesses. Science 2008, 319, 962–965.
- Kehl-Fie, T.E.; Zhang, Y.; Moore, J.L.; Farrand, A.J.; Hood, M.I.; Rathi, S.; Chazin, W.J.; Caprioli, R.M.; Skaar, E.P. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun. 2013, 81, 3395–3405.
- Chen, C.; Hooper, D.C. Intracellular accumulation of staphylopine impairs the fitness of Staphylococcus aureus cntE mutant. FEBS Lett. 2019, 593, 1213–1222.
- Grim, K.P.; Radin, J.N.; Solórzano, P.K.P.; Morey, J.R.; Frye, K.A.; Ganio, K.; Neville, S.L.; McDevitt, C.A.; Kehl-Fie, T.E. Intracellular Accumulation of Staphylopine Can Sensitize Staphylococcus aureus to Host-Imposed Zinc Starvation by Chelation-Independent Toxicity. J. Bacteriol. 2020, 202, 00014–00020.
- Luo, S.; Ju, Y.; Zhou, J.; Gu, Q.; Xu, J.; Zhou, H. Crystal structure of CntK, the cofactor-independent histidine racemase in staphylopine-mediated metal acquisition of Staphylococcus aureus. Int. J. Biol. Macromol. 2019, 135, 725–733.
- Luo, Z.; Luo, S.; Ju, Y.; Ding, P.; Xu, J.; Gu, Q.; Zhou, H. Structural insights into the ligand recognition and catalysis of the key aminobutanoyltransferase CntL in staphylopine biosynthesis. FASEB J. 2021, 5, 21575.
- Abideen, Z.U.; Ahmad, A.; Usman, M.; Majaz, S.; Ali, W.; Noreen, S.; Mahmood, T.; Nouroz, F. Dynamics and conformational propensities of staphylococcal CntA. J. Biomol. Struct. Dyn. 2021, 39, 4923–4935.
- Vinué, L.; Hooper, D.C. Rsp activates expression of the Cnt system in Staphylococcus aureus. BMC Microbiol. 2020, 20, 327.
- Kotecka, K.; Kawalek, A.; Kobylecki, K.; Bartosik, A.A. The AraC-Type Transcriptional Regulator GliR (PA3027) Activates Genes of Glycerolipid Metabolism in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 5066.
- Zhang, Y.; Zhang, H.; Wang, Z.; Wu, Z.; Wang, Y.; Tang, N.; Xu, X.; Zhao, S.; Chen, W.; Ji, Q. Programmable adenine deamination in bacteria using a Cas9-adenine-deaminase fusion. Chem. Sci. 2020, 6, 1657–1664.
