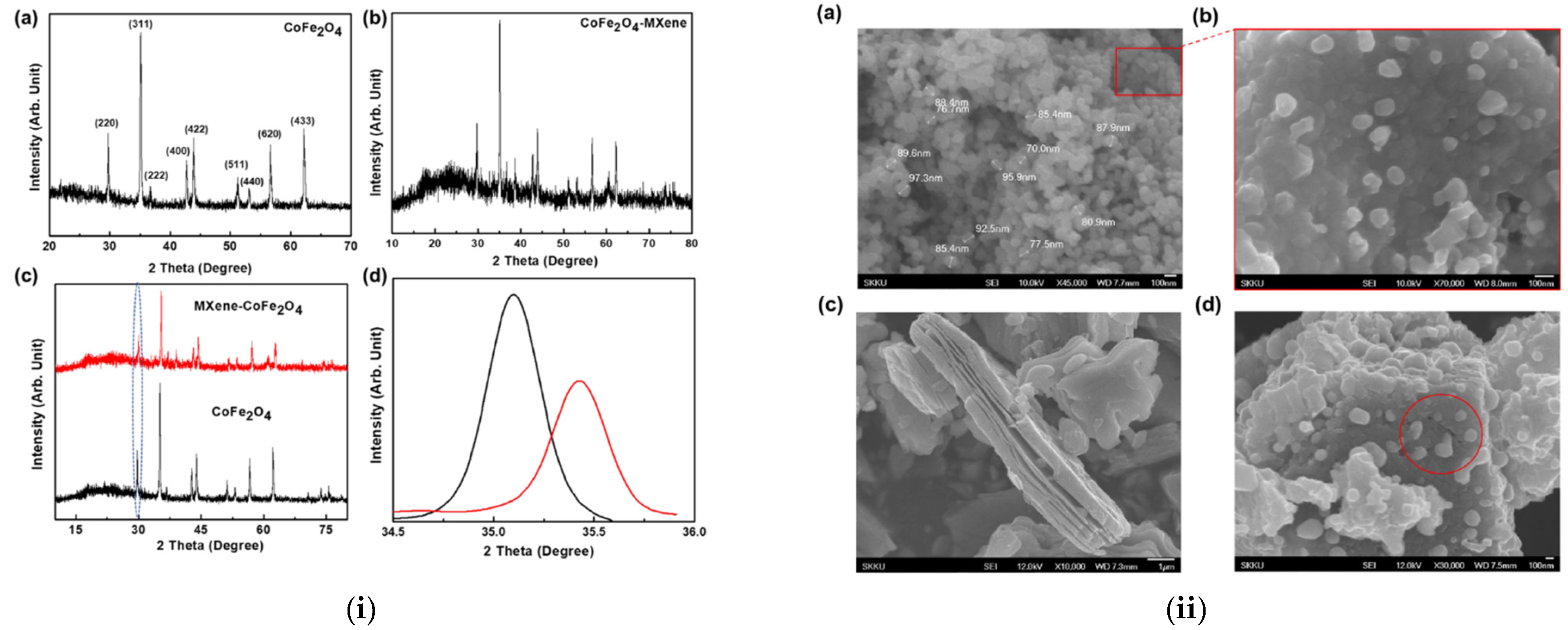
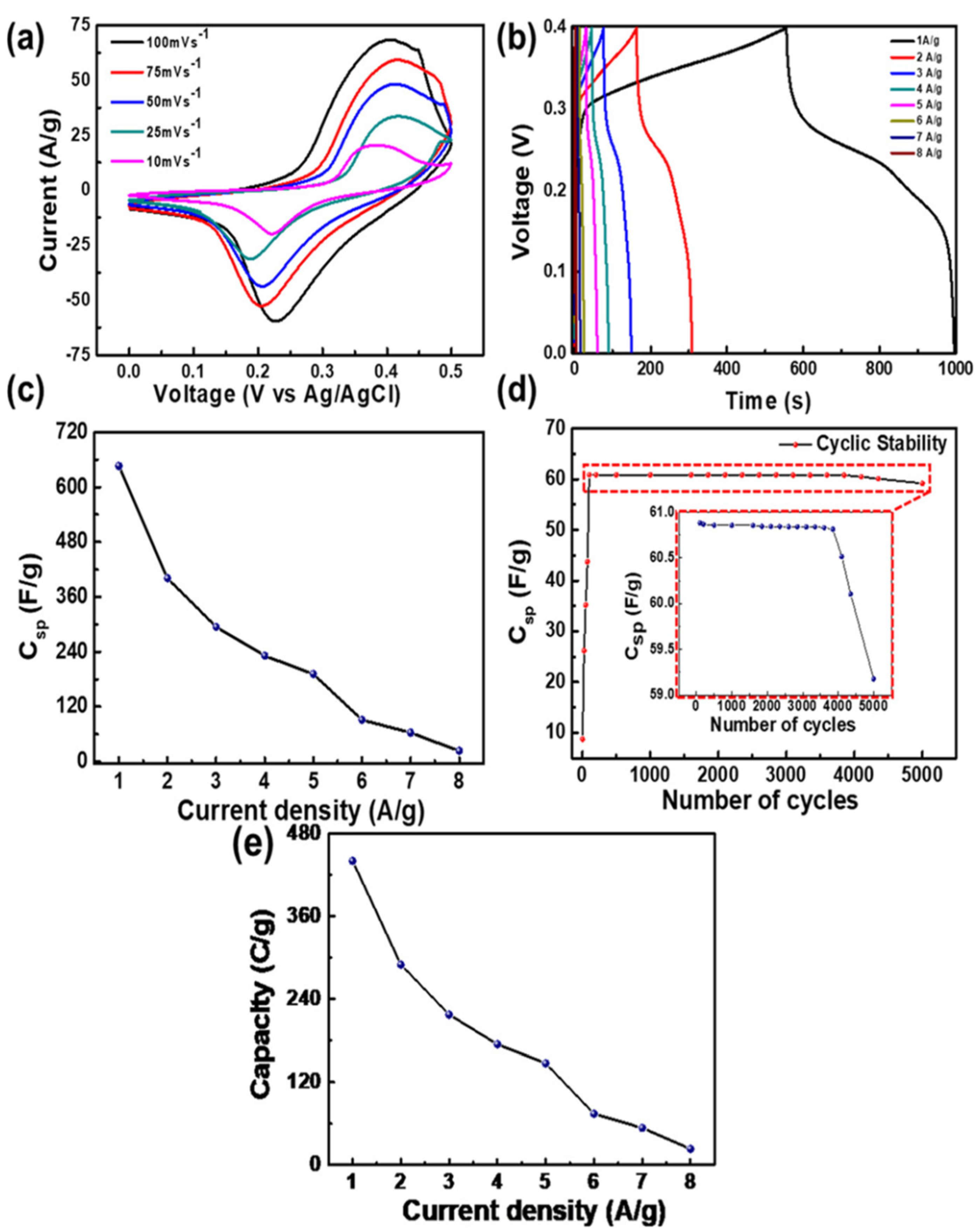
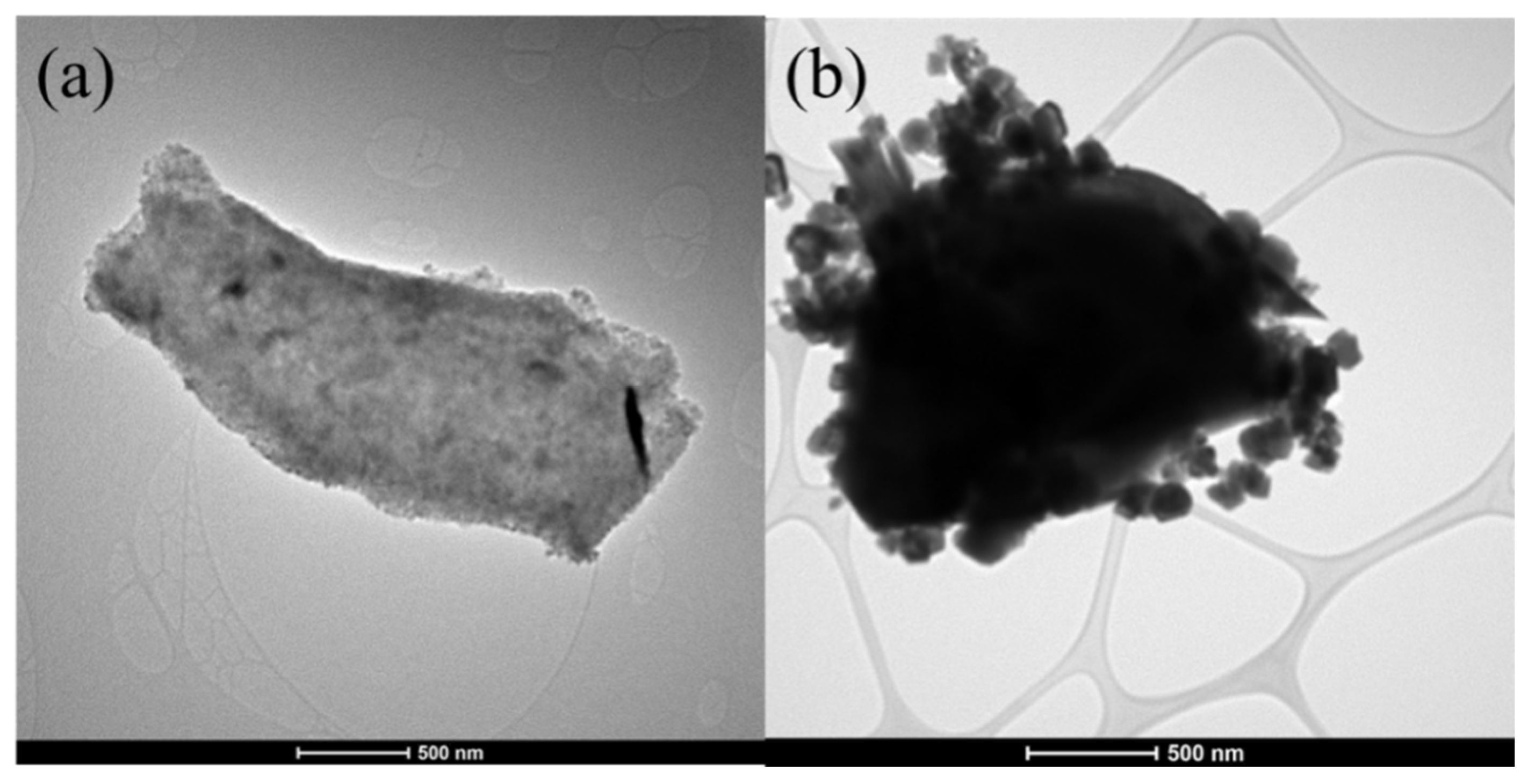
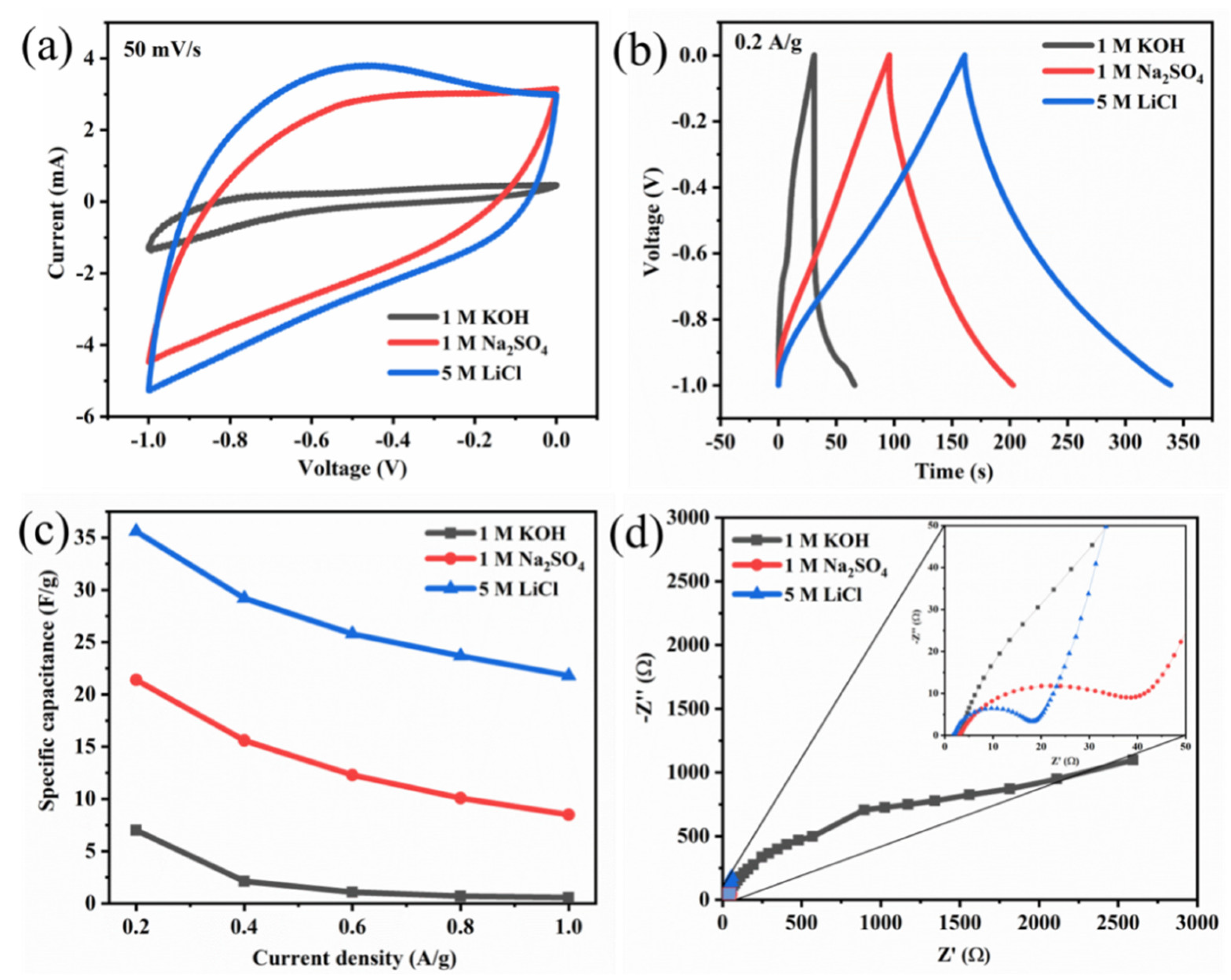
MXene has been identified as a new emerging material for various applications including energy storage, electronics, and bio-related due to its wider physicochemical characteristics. Further the formation of hybrid composites of MXene with other materials makes them interesting to utilize in multifunctional applications. The selection of magnetic nanomaterials for the formation of nanocomposite with MXene would be interesting for the utilization of magnetic characteristics along with MXene. However, the selection of the magnetic nanomaterials is important, as the magnetic characteristics of the ferrites vary with the stoichiometric composition of metal ions, particle shape and size. The selection of the electrolyte is also important for electrochemical energy storage applications, as the electrolyte could influence the electrochemical performance. Further, the external magnetic field also could influence the electrochemical performance.
- MXene
- ferrites
- supercapacitor applications
1. Introduction
2. MXene/Ferrite Electrode for Supercapacitor Applications
This entry is adapted from the peer-reviewed paper 10.3390/mi13101792
References
- Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K.K. MXene-Based Electrodes for Supercapacitor Energy Storage. Energy Fuels 2022, 36, 2390–2406.
- Zhan, C.; Naguib, M.; Lukatskaya, M.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D.E. Understanding the MXene Pseudocapacitance. J. Phys. Chem. Lett. 2018, 9, 1223–1228.
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a Charge Storage Host. Acc. Chem. Res. 2018, 51, 591–599.
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494.
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005.
- Mu, X.; Wang, D.; Du, F.; Chen, G.; Wang, C.; Wei, Y.; Gogotsi, Y.; Gao, Y.; Dall’Agnese, Y. Revealing the Pseudo-Intercalation Charge Storage Mechanism of MXenes in Acidic Electrolyte. Adv. Funct. Mater. 2019, 29, 1902953.
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098.
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and Conductive MXene Films and Nanocomposites with High Capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681.
- Kurra, N.; Ahmed, B.; Gogotsi, Y.; Alshareef, H.N. MXene-on-Paper Coplanar Microsupercapacitors. Adv. Energy Mater. 2016, 6, 1601372.
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. Synthesis and Characterization of Two-Dimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520.
- Zhu, Q.; Li, J.; Simon, P.; Xu, B. Two-Dimensional MXenes for Electrochemical Capacitor Applications: Progress, Challenges and Perspectives. Energy Storage Mater. 2021, 35, 630–660.
- Das, P.; Wu, Z.S. MXene for Energy Storage: Present Status and Future Perspectives. J. Phys. Energy 2020, 2, 032004.
- Ando, Y.; Okubo, M.; Yamada, A.; Otani, M. Capacitive versus Pseudocapacitive Storage in MXene. Adv. Funct. Mater. 2020, 30, 2000820.
- Xu, J.; Hu, X.; Wang, X.; Wang, X.; Ju, Y.; Ge, S.; Lu, X.; Ding, J.; Yuan, N.; Gogotsi, Y. Low-Temperature Pseudocapacitive Energy Storage in Ti3C2Tx MXene. Energy Storage Mater. 2020, 33, 382–389.
- Wang, T.; Zhao, J.; Qi, L.; Li, G.; Yang, W.; Li, Y. Ultrathin Graphdiyne Oxide-Intercalated MXene: A New Heterostructure with Interfacial Synergistic Effect for High Performance Lithium-Ion Storage. Energy Storage Mater. 2023, 54, 10–19.
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725.
- Nithya, V.D.; Sabari Arul, N. Progress and Development of Fe3O4 Electrodes for Supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778.
- Arun, T.; Kavin Kumar, T.; Udayabhaskar, R.; Morel, M.J.; Rajesh, G.; Mangalaraja, R.V.; Akbari-Fakhrabadi, A. Size Dependent Magnetic and Capacitive Performance of MnFe2O4 Magnetic Nanoparticles. Mater. Lett. 2020, 276, 128240.
- Nwodo, M.O.; Obodo, R.M.; Nwanya, A.C.; Ekwealor, A.B.C.; Ezema, F.I. Recent Developments in Metal Ferrite Materials for Supercapacitor Applications. In Electrode Materials for Energy Storage and Conversion; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK, 2021; pp. 171–212.
- Arun, T.; Kavinkumar, T.; Udayabhaskar, R.; Kiruthiga, R.; Morel, M.J.; Aepuru, R.; Dineshbabu, N.; Ravichandran, K.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V. NiFe2O4 Nanospheres with Size-Tunable Magnetic and Electrochemical Properties for Superior Supercapacitor Electrode Performance. Electrochim. Acta 2021, 399, 139346.
- Kumar, S.; Kumar, P.; Singh, N.; Kansal, M.K.; Kumar, A.; Verma, V. Highly Efficient and Sustainable MXene Based Heterostructure Composites Filled with Ferrites and MWCNTs to Mitigate the Radiation Interference in X-Band Frequency Region. Mater. Sci. Eng. B 2022, 282, 115798.
- Ayman, I.; Rasheed, A.; Ajmal, S.; Rehman, A.; Ali, A.; Shakir, I.; Warsi, M.F. CoFe2O4Nanoparticle-Decorated 2D MXene: A Novel Hybrid Material for Supercapacitor Applications. Energy Fuels 2020, 34, 7622–7630.
- Arun, T.; Dhanabalan, S.S.; Udayabhaskar, R.; Ravichandran, K.; Akbari-Fakhrabadi, A.; Morel, M.J.; Arun, T.; Dhanabalan, S.S.; Akbari-Fakhrabadi, A.; Morel, M.J. Magnetic Nanomaterials for Energy Storage Applications; Springer: Cham, Switzerland, 2022; pp. 131–150.
- Guo, Z.; Ren, P.; Lu, Z.; Hui, K.; Yang, J.; Zhang, Z.; Chen, Z.; Jin, Y.; Ren, F. Multifunctional /Cellulose Nanofiber Composite Films with Asymmetric Layered Architecture for High-Efficiency Electromagnetic Interference Shielding and Remarkable Thermal Management Capability. ACS Appl. Mater. Interfaces 2022, 14, 41468–41480.
- Yang, X.; Liu, Y.; Hu, S.; Yu, F.; He, Z.; Zeng, G.; Feng, Z.; Sengupta, A. Construction of Fe3O4@MXene Composite Nanofiltration Membrane for Heavy Metal Ions Removal from Wastewater. Polym. Adv. Technol. 2021, 32, 1000–1010.
- Xu, J.; Zeng, G.; Lin, Q.; Gu, Y.; Wang, X.; Feng, Z.; Sengupta, A. Application of 3D Magnetic Nanocomposites: MXene-Supported Fe3O4@CS Nanospheres for Highly Efficient Adsorption and Separation of Dyes. Sci. Total Environ. 2022, 822, 153544.
- Zhang, Z.; Wang, B.; Han, R.; Jin, F.; Zhang, N.; Zhang, T.; Wang, D. 3D 3O4 as Cathode Additive for Rechargeable Lithium−Sulfur Batteries. Adv. Energy Sustain. Res. 2022, 3, 2100167.
- Thirumurugan, A.; Akbari-Fakhrabadi, A.; Joseyphus, R.J. Surface Modification of Highly Magnetic Nanoparticles for Water Treatment to Remove Radioactive Toxins. In Green Methods for Wastewater Treatment; Springer: Cham, Switzerland, 2020; pp. 31–54.
- Arun, T.; Mohanty, A.; Rosenkranz, A.; Wang, B.; Yu, J.; Morel, M.J.; Udayabhaskar, R.; Hevia, S.A.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; et al. Role of Electrolytes on the Electrochemical Characteristics of Fe3O4/MXene/RGO Composites for Supercapacitor Applications. Electrochim. Acta 2021, 367, 137473.
