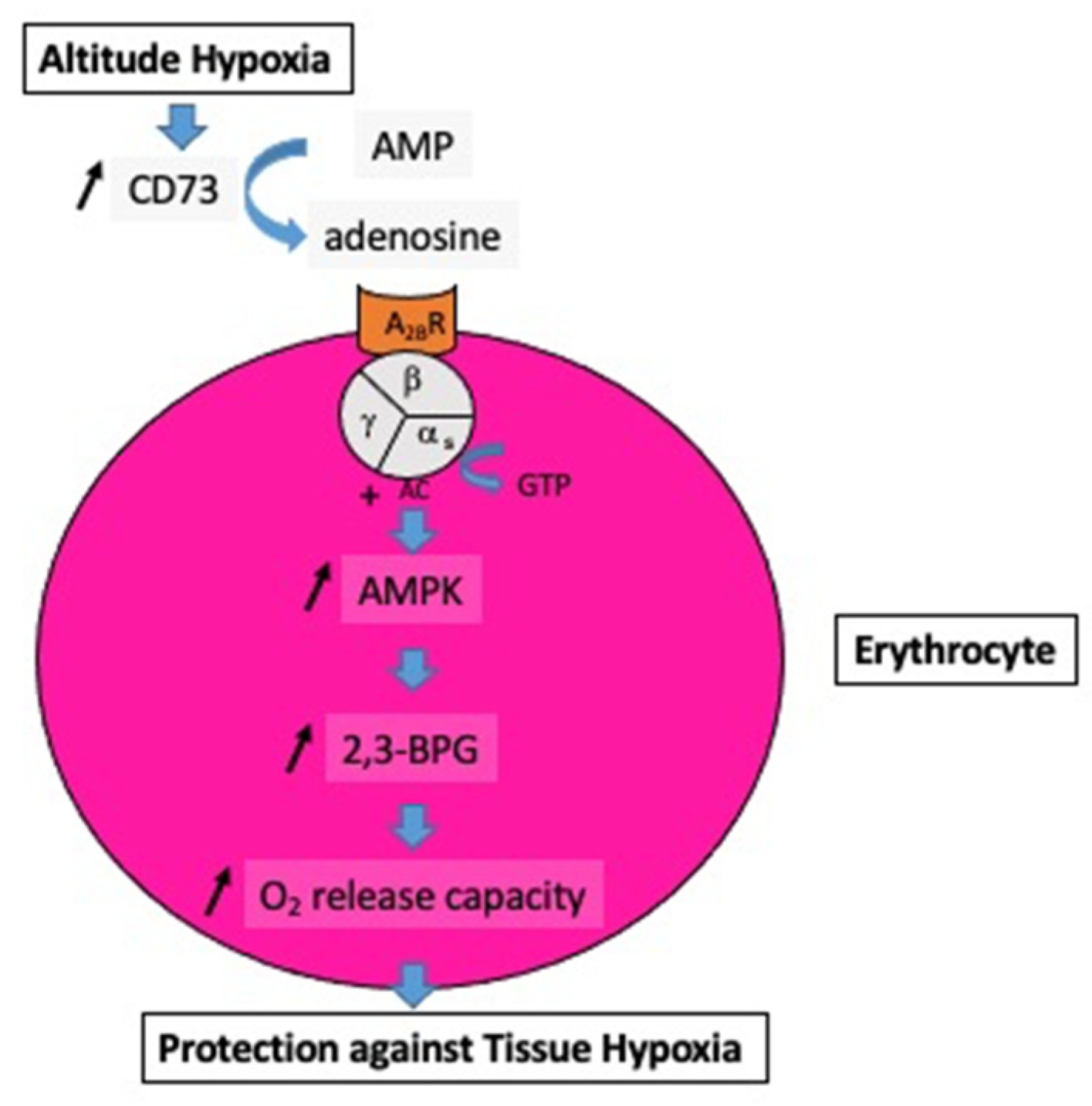
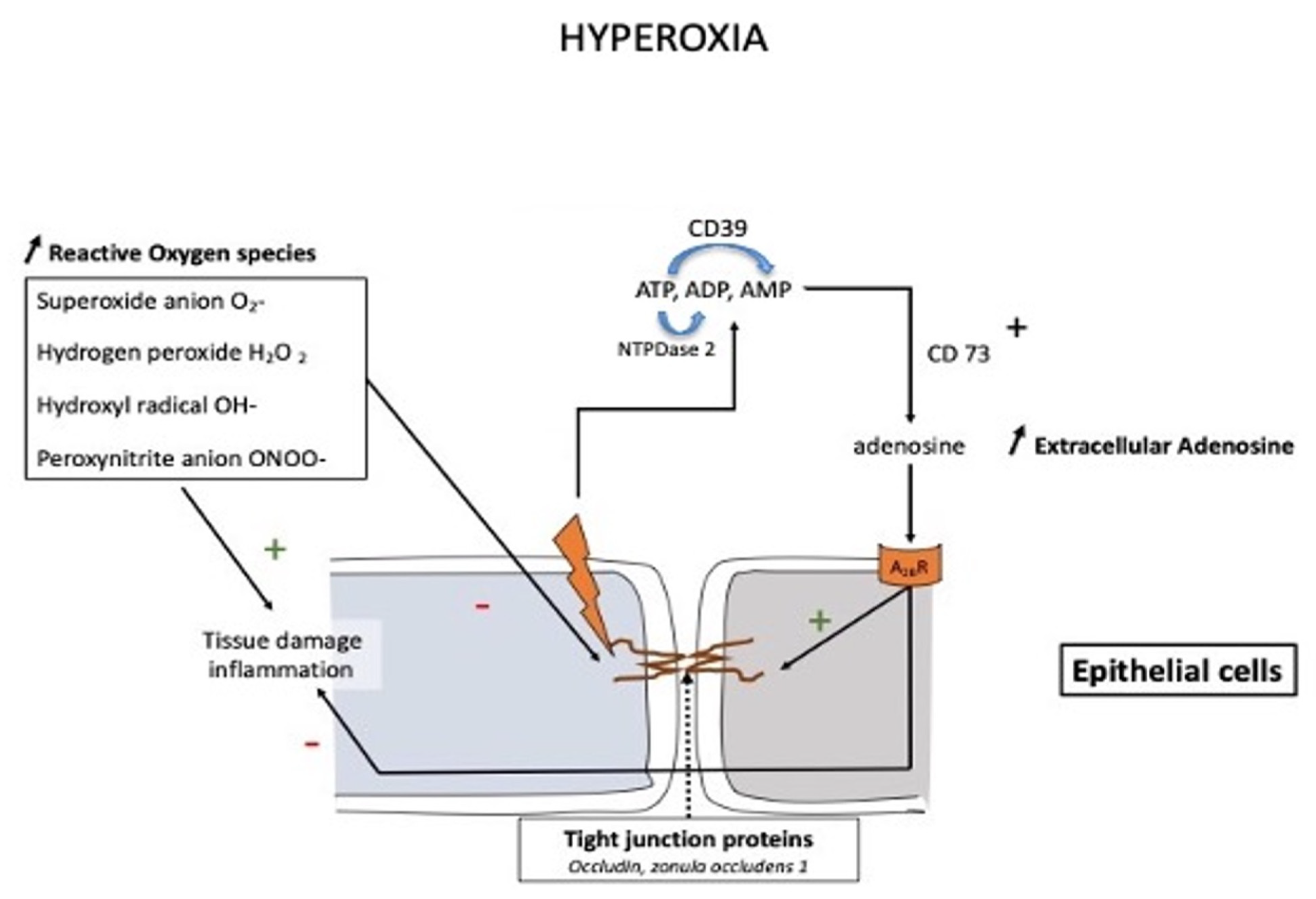
At high altitudes, the increased adenosine concentration contributes to brain protection against hypoxia through various mechanisms such as stimulation of glycogenolysis for ATP production, reduction in neuronal energy requirements, enhancement in 2,3-bisphosphoglycerate production, and increase in cerebral blood flow secondary to vasodilation of cerebral arteries. In the context of mountain illness, the increased level of A2AR expression leads to glial dysfunction through neuroinflammation and is involved in the pathogenesis of neurological disorders. Nonetheless, a high level of adenosine concentration can protect against high-altitude pulmonary edema via a decrease in pulmonary arterial pressure. The adenosinergic system is also involved in the acclimatization phenomenon induced by prolonged exposure to altitude hypoxia. During hyperoxic exposure, decreased extracellular adenosine and low A2A receptor expression contribute to vasoconstriction. The resulting decrease in cerebral blood flow is considered a preventive phenomenon against cerebral oxygen toxicity through the decrease in oxygen delivery to the brain. With regard to lung oxygen toxicity, hyperoxia leads to an increase in extracellular adenosine, which acts to preserve pulmonary barrier function. Changes in the adenosinergic system induced by exposure to extreme oxygen partial pressures frequently have a benefit in decreasing the risk of adverse effects.
- Adenosine
- hypoxia
- altitude
- hyperoxia
- diving
1. Introduction
1.1. Altitude Hypoxia
1.2. Acute Altitude Illness
1.3. Chronic Hypoxia: Life at Altitude
2. Hyperoxia
2.1. Cardio-Vascular Changes
2.2. Oxygen Toxicity
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092059
References
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular adenosine: A safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid. Redox. Signal. 2011, 15, 2221–2234.
- Eltzschig, H.K.; Karhausen, J.; Kempf, V.A. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2006, 354, 975–977.
- Joulia, F.; Coulange, M.; Lemaitre, F.; Costalat, G.; Franceschi, F.; Gariboldi, V.; Nee, L.; Fromonot, J.; Bruzzese, L.; Gravier, G.; et al. Plasma adenosine release is associated with bradycardia and transient loss of consciousness during experimental breath-hold diving. Int. J. Cardiol. 2013, 168, e138–e141.
- Bruzzese, L.; Rostain, J.C.; Née, L.; Condo, J.; Mottola, G.; Adjriou, N.; Mercier, L.; Berge-Lefranc, J.L.; Fromonot, J.; Kipson, N.; et al. Effect of hyperoxic and hyperbaric conditions on the adenosinergic pathway and CD26 expression in rat. J. Appl. Physiol. 2015, 119, 140–147.
- Fromonot, J.; Chaumet, G.; Gavarry, O.; Rostain, J.C.; Lucciano, M.; Joulia, F.; Brignole, M.; Deharo, J.C.; Guieu, R.; Boussuges, A. Hyperoxia Improves Hemodynamic Status During Head-up Tilt Testing in Healthy Volunteers: A Randomized Study. Medicine 2016, 95, e2876.
- Morote-Garcia, J.C.; Rosenberger, P.; Kuhlicke, J.; Eltzschig, H.K. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 2008, 111, 5571–5580.
- Hellsten, Y.; Nyberg, M.; Mortensen, S.P. Contribution of intravascular versus interstitial purines and nitric oxide in the regulation of exercise hyperaemia in humans. J. Physiol. 2012, 590, 5015–5023.
- Peacock, A.J. ABC of oxygen: Oxygen at high altitude. BMJ 1998, 317, 1063–1066.
- Brugniaux, J.V.; Hodges, A.N.; Hanly, P.J.; Poulin, M.J. Cerebrovascular responses to altitude. Respir. Physiol. Neurobiol. 2007, 158, 212–223.
- Winn, H.R.; Rubio, R.; Berne, R.M. Brain adenosine concentration during hypoxia in rats. Am. J. Physiol. 1981, 241, H235–H242.
- Le, G.; Essackjee, H.; Ballard, H. Intracellular adenosine formation and release by freshly-isolated vascular endothelial cells from rat skeletal muscle: Effects of hypoxia and/or acidosis. Biochem. Biophys. Res. Commun. 2014, 450, 93–98.
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317.
- Sumi, Y.; Woehrle, T.; Chen, Y.; Yao, Y.; Li, A.; Junger, W.G. Adrenergic receptor activation involves ATP release and feedback through purinergic receptors. Am. J. Physiol. Physiol. 2010, 299, C1118–C1126.
- Song, A.; Zhang, Y.; Han, L.; Yegutkin, G.G.; Liu, H.; Sun, K.; D’Alessandro, A.; Li, J.; Karmouty-Quintana, H.; Iriyama, T.; et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat. Commun. 2017, 8, 14108.
- Liu, H.; Zhang, Y.; Wu, H.; D’Alessandro, A.; Yegutkin, G.G.; Song, A.; Sun, K.; Li, J.; Cheng, N.Y.; Huang, A.; et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation 2016, 134, 405–421.
- Görlach, A. Control of adenosine transport by hypoxia. Circ. Res. 2005, 97, 1–3.
- Laborit, H.; Bonifacj, J.F. Action of adenosine on energy metabolism and on glucose-6-phosphate dehydrogenase in rat brains. Res. Commun. Chem. Pathol. Pharmacol. 1984, 44, 123–130.
- West, J.B. High-altitude medicine. Lancet Respir. Med. 2015, 3, 12–13.
- Salys, J.; Kratzer, A.; Zamora, M.; Taraseviciene-Stewart, L. Hypoxia-mediated alterations in adenosine receptor expression in rat lung. Eur. Respir. J. 2011, 38, 333.
- Sharma Kandel, R.; Mishra, R.; Gautam, J.; Alaref, A.; Hassan, A.; Jahan, N. Patchy vasoconstriction versus inflammation: A debate in the pathogenesis of high altitude pulmonary edema. Cureus 2020, 12, e10371.
- Calbet, J.A.; Boushel, R.; Robach, P.; Hellsten, Y.; Saltin, B.; Lundby, C. Chronic hypoxia increases arterial blood pressure and reduces adenosine and ATP induced vasodilatation in skeletal muscle in healthy humans. Acta Physiol. 2014, 211, 574–584.
- Waring, W.; Thomson, A.; Adwani, S.; Rosseel, A.; Potter, J.; Webb, D.; Maxwell, S. Cardiovascular effects of acute oxygen administration in healthy adults. J. Cardiovasc. Pharmacol. 2003, 42, 245–250.
- Molenat, F.; Boussuges, A.; Grandfond, A.; Rostain, J.C.; Sainty, J.M.; Robinet, C.; Galland, F.; Meliet, J.L. Modifications of cardiovascular function secondary to hyperbaric hyperoxia in healthy volunteers: An Echocardiographic and Doppler study. Clin. Sci. 2004, 106, 389–395.
- Gole, Y.; Gargne, O.; Coulange, M.; Steinberg, J.G.; Bouhaddi, M.; Jammes, Y.; Regnard, J.; Boussuges, A. Hyperoxia-induced alterations in cardiovascular function and autonomic control during return to normoxic breathing. Eur. J. Appl. Physiol. 2011, 111, 937–946.
- Harten, J.M.; Anderson, K.J.; Angerson, W.J.; Booth, M.G.; Kinsella, J. The effect of normobaric hyperoxia on cardiac index in healthy awake volunteers. Anaesthesia 2003, 58, 885–888.
- Rossi, P.; Boussuges, A. Hyperoxia-induced arterial compliance decrease in healthy man. Clin. Physiol. Funct. Imag. 2005, 25, 10–15.
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide anions and hyperoxia inactivate endothelium derived relaxing factor. Am. J. Physiol. 1986, 250, H822–H827.
- Welsh, D.G.; Jackson, W.F.; Segal, S.S. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: A role for L-type Ca2+ channels. Am. J. Physiol. 1998, 274, H2018–H2024.
- Scott, A.; Fruttiger, M. Oxygen-induced retinopathy: A model for vascular pathology in the retina. Eye 2010, 24, 416–421.
- Zhang, S.; Li, H.; Li, B.; Zhong, D.; Gu, X.; Tang, L.; Wang, Y.; Wang, C.; Zhou, R.; Li, Y.; et al. Adenosine A1 Receptors Selectively Modulate Oxygen-Induced Retinopathy at the Hyperoxic and Hypoxic Phases by Distinct Cellular Mechanisms. Investig. Ophthalmol Vis. Sci. 2015, 56, 8108–8119.
- Ottolenghi, S.; Sabbatini, G.; Brizzolari, A.; Samaja, M.; Chiumello, D. Hyperoxia and oxidative stress in anesthesia and critical care medicine. Minerva Anestesiol. 2020, 86, 64–75.
- Arieli, R.; Arieli, Y.; Daskalovic, Y.; Eynan, M.; Abramovich, A. CNS oxygen toxicity in closed-circuit diving: Signs and symptoms before loss of consciousness. Aviat. Space Environ. Med. 2006, 77, 1153–1157.
- Jones, M.W.; Brett, K.; Han, N.; Wyatt, H.A. Hyperbaric Physics; StatPearls Publishing: Treasure Island, FL, USA, 2017.
