Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Ceramics
Bricks are typically made through the high-temperature firing process or by stabilizing the mixture with binders such as lime and cement. These bricks have a large carbon footprint and high levels of grey energy. In many parts of the world, the excessive use of clay as a natural raw material for the production of conventional bricks will lead to its scarcity. The mixing of clay with lignocellulosic ash during brick manufacturing leads to a better and more reliable solution that conserves scarce natural resources and reduces the impact of environmental pollution.
- fired bricks
- unfired bricks
- geopolymer bricks
1. Unfired Clay Bricks Reinforced by Lignocellulosic Biomass Ash
Unfired clay brick construction has several benefits, such as the accessibility of raw materials, fire resistance, favorable climatic performance, low cost, and low energy consumption. These benefits combine to make these blocks a desirable building material. However, the low durability, low tensile strength, low impact resistance, low abrasion resistance, and high-water absorption capacity of these bricks are what restrict their use. Many materials, such as cement, lime, fly ash, gypsum, and bitumen, were used for soil stabilization to overcome these difficulties. In the majority of studies, cement and lime are the main components used to create stabilized soil blocks. Currently, the use of cement and lime can be offset by combining them with other wastes, such as lignocellulosic biomass ash, which is the main component of stabilized earth blocks (Table 3). Ref. [77] investigated the mechanical properties of raw earth blocks stabilized with 4% and 10% ordinary Portland cement (OPC) and altered with 4%, 6%, and 8% sugarcane bagasse ash (SBA). Except for the addition of 4% SBA, where a slight loss of strength of 3.86% could be felt, earth blocks stabilized with 4% cement show a steady increase in strength with increasing SBA content. However, a gain of 2.32% and 13.9% was observed when adding 6% and 8% SBA (2.56 MPa and 2.95 MPa, compared to 2.59 MPa of the control block). For 10% cement stabilized blocks, incremental additions of 4%, 6%, and 8% SBA led to steady increases in compressive strength of 1.48%, 2.77%, and 7.93%. Adding SBA at a lower cement content led to a greater increase in compressive strength. Adding 8% SBA was the only way to achieve a compressive strength that complied with standards for the earth block stabilized with 4% cement. However, the compressive strength was attained for blocks stabilized with 10% cement without the addition of SBA. For water absorption, the addition of SBA to cement yielded a slight increase in the water absorption of the blocks. This water absorption was higher at a cement content of 10% than at a cement content of 4% which proves that the addition of SBA is more effective at lower cement contents. In a related study, Ref. [78] prepared two stabilized earth blocks with cement contents of 6% and 12% and added sugar bagasse ash (SBA) at contents of 0%, 2%, 4%, and 8%, respectively. The choice of cement content is due to the granulometry of the soil used, thus making their blocks fall into the category of cement-bound materials. The amount of cement needed to stabilize the raw earth bricks depends significantly on the type of earth that will be used to create the specimens. While a minimum of 6% cement could be used if the earth had a significant amount of clay in it, 4% cement can produce high-performance blocks if the soil is too sandy. Strengthening to 8% SBA resulted in a maximum increase in block strength of up to 54% for bricks stabilized with 6% cement. Despite the increase in strength due to the progressive addition of SBA with 6% cement, the average values of this strength did not exceed 1.54 MPa at 28 days. For the blocks stabilized at 12% cement, an average value of the compressive strength at 28 days was 3.13 Mpa at 0% SBA. The addition of SBA results in a reduction in the mechanical strength, but this reduction is less significant for the addition of 8% SBA, or a value of 2.89 MPa was measured. Ref. [79] investigated the stabilization of residual soils using 4% to 8% cement and rice husk ash (RHA) at percentages ranging from 5% to 25% by step of 5%. When comparing the compressive strength results of stabilization of earthen blocks by adding (RHA) to cement compared to cement alone, it was seen an increase in mechanical strength. The use of 4% cement alone for stabilizing the earthen blocks leads to a compressive strength of 0.882 MPa. However, the addition of 5%, 10%, 15%, 20% and 25% RHA generates a compressive strength of 1.654, 3.154, 3.309, 3.011 and 1.187 MPa, respectively. As a result, the formula with 15% to 20% of RHA increases the compressive strength significantly when compared to the cement alone, while above this value range, the resistance significantly decreases.
In the same way, Ref. [80] considered a combination of lime, cement, and SBA for stabilizing the compacted soil blocks. The mixture was designated as NA, CAL, CEM, and CALBA, which refer to the compacted soil blocks, respectively, when it contained no additives, 10% lime, 10% cement, and a combination of 10% lime and 10% sugarcane bagasse ash (SBA). Results of compressive strength tests show that adding cement and lime to the clay matrix increased the clay matrix’s compressive strength. The cement mixture (CEM) showed a compressive strength of 12.7 MPa, while the lime mixture (CAL) showed a strength of 5.55 MPa. When SBA is added to lime in an equal amount (CALBA), the compressive strength increases by almost 40% and is measured at 7.7 MPa. This growth of compressive strength could be attributed to the progressive densification of the clay matrix and more precisely to hydration and pozzolanic reactions. For his part, Ref. [81] calculated the amount of lime required to stabilize the soil using the pH method developed by [82]. The optimal initial lime was found to be 6%. Moreover, he concluded that the stabilization could be achieved with three different sugarcane bagasse ash (BA) contents; 4%, 6%, and 8%, respectively. For this, a linear relationship between compressive strength and the percentage of BA was found, which was supported by a fit with a regression factor R2 = 0.94. By adding 8% BA, this strength increased from 1.687 MPa to 1.87 MPa, or by almost 11%. Ref. [82] investigated the possibility of using lime and rice husk (RHA) for the development of new unfired brick or compressed stabilized earth (CSE) forms. To reduce the effect of shrinkage, clay as the matrix material of the brick was mixed with sand. A standard Proctor compaction test of soil and sand mixture was performed to obtain an optimal value of the sand used according to their compaction characteristics. Thus, a maximum dry density value among the test mixture was obtained for a 70% soil–30% sand mixture. The amount of lime required for stabilization (LRS) was determined according to the method developed by [82]. Accordingly, 5% lime was found to be the optimum amount of lime to stabilize the tested soil-sand mixtures. The ratio of lime and RHA was selected as 1:1 (5%:5%), 1:2 (5%:10%), 1:3 (5%:15%), 2:1 (10%:5%). It was observed that the addition of lime and RHA increases the compressive strength. This strength reaches a maximum value of 15.5 Mpa and 16.1 Mpa with a lime/RHA ratio of 1:1 for the treated clay sample and the treated clay/sand mixture sample, respectively. After this limit, the compressive strength decreases. The reason for the improved performance of clay mixed with sand is that the finer particles of clay will fill the voids of the coarser particles of sand. This would result in denser, stronger, waterproof soil mixtures which result in a decrease in water absorption. The BIS specification in IS 1725 (BIS 1982) provides for two classes of stabilized blocks, namely class 20 with a minimum allowable strength of 1.96 MPa and class 30 with a minimum allowable strength of 2.94 MPa [83]. The ideal mechanical strength for each of the aforementioned studies is depicted in Figure 8.
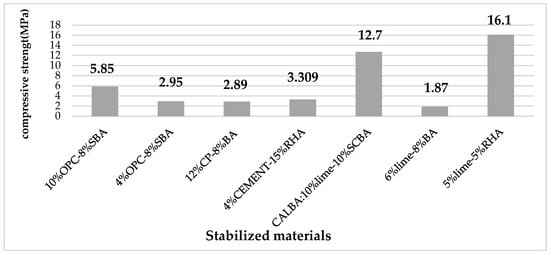
The compressive strengths of all unfired clay bricks stabilized by the addition of cement/lime with lignocellulosic ash percentages, as shown in Figure 8, are higher than those of class 30, except for the study [81], where the strength corresponding to 6% lime stabilized mud block was insufficient to meet the minimum requirements of a class 20 block. According to the relationship equation developed from the fit between compressive strength and bagasse ash (BA) content, a minimum BA content of 9.42% was discovered to be necessary to achieve a strength of 1.96 MPa for Class 20 blocks [81]. In comparison to cement, this diagram demonstrates that lime is the more effective stabilizing agent. Indeed, the lignocellulosic biomass ashes are pozzolans rich in siliceous and silicoaluminous materials by themselves in powder form and in the presence of water. The silica and alumina of the pozzolans react with the calcium hydroxide continuously in the lime. Numerous hydrated calcium silicates (C-S-H), hydrated calcium aluminates, and hydrated calcium silico-aluminates (C-A-S-H) are precipitated as a result of this pozzolanic reaction. These gels formed could fulfill the voids, and give a much more impermeable structure, much denser which also translates into a gain of appreciable strength. However, several parameters could affect the mechanical properties of the pozzolanic reaction. The size of the particles is a crucial parameter. Indeed, the mixture of lime with pozzolans allows the porosity lowering of the open pores when the particles of lignocellulosic ash used are sufficiently fine. Whereas a lack of fine particles could limit both: the pozzolanic reaction and the compressive strength of the stabilized earth bricks [85]. Additionally, the presence of water increases the amount of gel that forms, favoring particle binding and increasing compressive strength [86].
The presence of carbon in the mineralogical composition may be another cause of the low pozzolanic activity in the binder-ash mixture [87]. Indeed, as a tetravalent element, carbon can both give or receive electrons. It will tend to give electrons when bonding with elements with more than four valence electrons. When there is an excess of carbon, the tendency is to form planar structures because of the ease of double bonds. The interplanar distance is therefore important and can induce water absorption decreasing the amount of water available for the hydration process of the pozzolanic reaction. This might subsequently prevent the synthesis of gels such as C-S-H and C-A-H. The color of lignocellulosic biomass ashes gives us an idea of the presence or not of carbon in its composition.
Table 3. Studies on the manufacturing of unfired bricks using waste lignocellulosic biomass ash.
| Article | Stabilized Materials | Clay | Reinforcement Rate | Moisture of Molding | Forming Technique | Curing Conditions | Characteristics of Bricks |
|---|---|---|---|---|---|---|---|
| [77] | Cement (OPC)-sugarcane Bagasse. Ash (SBA) 300 µm | Solid | 4–10% OPC 4–6–8% SBA |
12% | Compression | Moisture cured for a period of 28 days by sprinkling water and converting it with plastic gunny bags | Water absorption Compressive strength |
| [78] | Limestone Portland Cement-sugarcane ash bagasse (SBA) |
Sandy earth from the region of Portugal/Kaolin (1–7) | 6% and 12% of cement 0%, 2%, 4% and 8% of SBA |
The amount of water is adjusted for each series and it increased as the amount of SBA increased | Manual press | Room with controlled ventillation and no direct sun-light | Water absorption Compressive strength |
| [81] | Hydrated lime-Bagasse-ash (BA) | Virgin soil | 6% of lime with 0, 4, 6 and 8% of BA | 12% | The soil blocks were cast to a fixed density of 18.5 kN/m3 | Sprinkle cured for a period of 28 days and covered with plastic gunny bags | Compressive strength Water absorption |
| [83] | Hydrated lime (RHA) | Clay soils 70–30% sand | Lime-RHA 1:1 (5–5%), 1:2 (5–10%), 1:3 (5–15%), 2:1 (10–5%), and 3:1 (15–5%) | 19% | Hand-operated compression machine of 15 MPa | Dried at humidity and room temperature (30 °C) for 28 days by covering them with a plastic sheet | Water absorption Compressive strength |
| [79] | Rice Husk in the incinerator ordinary Portland cement | Residual granite soil | 4% to 8% of cement and 0% to 25% rice husk ash | - | Compacted with mechanical rammer of 2.5 kg | Cured in plastic bag for 7 days after 7 days of moist-curing. The specimen was then soaked for 7 days in water and the other specimen continued to cure until its old was 14 days | Compressive strength |
| [88] | NaOH 8M, KOH 8 M, Na2SiO3 8 M and KOH-K2SiO3 8 M | Calcined clays (CC (150 µm) Olive pomace fly ash (OPFA 150 µm) |
70% CC–30% OPFA | Liquid/solid ratio = 0.4 | Precursors cures at 60 °C and 98% RH for 24 h. Then specimens unmolded and stored under ambient conditions (21 ± 2 °C and 58 ± 2%RH) for 28 days | Bulk density compressive strength Thermal conductivity Loss of ignition |
|
| [89] | KOH from the calcination of olive biomass ash (OBFA) | Ceramic industry (chamotte) residue 250 µm | 10%, 20%, 30% and 40% of OBFA | 15% and 20% | Compaction pressure of 50 MPa | In an oven at 85 ± 5 °C temperature for 24 h | Weight loss Water absorption Boiling-water absorption Linear shrinkage Bulk density Open porosity Compressive strength |
| [90] | KOH from the biomass bottom ash of almond husk and alpeorujo combustions (BBA 0.25 mm) | Chamotte 0.25 mm | 10% to 100% of BBA with increases of 10% | 20% | - | Dried at room temperature (20 ± 2 °C) for 24 h and at 90 ± 2 °C for another 24 h | Compressive strength Capillarity water absorption Cold water absorption Boiling water absorption Bulk density Open porosity |
| [91] | Biomass bottom ash residue (BBA) from the combustion of a mix of olive and pine pruning <150 µm-sodium hydroxide 8 M | Metakaolin (MK) for different Spanish clays; Black clay(BC), Yellow clay (YC), White clay (WC) and red clay (RC) 150 µm | 33.3% of each ingredient | Water/binder = 0.6 | - | The specimens were cured at 60 °C and in a saturated atmosphere for 24 h. After this period, the samples were removed from the mold and kept at room temperature until 28 days | Bulk density Water absorption Apparent porosity Compressive strength Conductivity |
The stabilization of unfired bricks by chemical binders developed by alkaline activation to produce a polymerization reaction, responds perfectly to the recent challenges inherent to sustainable production. The most popular precursors used in the synthesis of geopolymers at the moment are natural clays that have undergone a thermal transformation to become calcined clays and lignocellulosic biomass ashes. The precursors of alkali activation of aluminosilicates during the geopolymerization reaction are sodium aluminates, alkali silicates, hydroxides, carbonates, and other additives. The mixture of silicate solution (Na2SiO3 or K2SiO3) and hydroxides (NaOH or KOH) is the most widely used activation solution [88] studied the feasibility of using olive pomace (OPFA) as an alkaline precursor in the manufacture of geopolymer bricks. Different samples were developed using alkaline activation solutions such as sodium hydroxide solution NaOH (8M) or potassium hydroxide solution KaOH (8M), or a mixture of alkaline hydroxide and alkaline silicate solution (NaOH-Na2SiO3) and (KOH-K2SiO3) with 70% calcined clay (CC) and 30% OPFA as aluminosilicate sources, to support this proposal. A control brick was made using only water during molding with the 70% CC–30% OPFA mixture. The results showed that OPFA can be used as an alkaline activator, presenting mechanical properties slightly lower than those obtained when adding alkaline hydroxide activation solutions. The control geopolymers have a compressive strength of 1.3 MPa. However, the use of alkali hydroxides leads to an increase in compressive strength to 3.0 and 3.9 MPa for sodium and potassium hydroxides, respectively. These values increase to 5.3 MPa and 9.0 MPa when sodium and potassium silicate solutions are used, respectively. However, the best thermal insulation properties were obtained for the control geopolymers. In fact, the use of activation solutions favors the densification of the unfired bricks, which increases the thermal conductivity values. Moreover, Carrillo-Beltran et al. [89] discussed the viability of producing a geopolymer from chamotte, a byproduct of the brick industry, and olive biomass fly ash (OBFA), which is produced by calcining olive pomace (OP) and dry olive cake (DO). These ashes are composed mainly of potassium about 53% (Table 4), crystalline and amorphous constituents, and residual unburned carbon. Calcination of these ashes was carried out to promote their decarbonization while potassium oxide (K2O) and carbon dioxide should be released. When potassium oxide is released, it dissolves in water and produces potassium hydroxide (KOH), the alkaline activation solution that leads to the formation of geopolymerization. Different amounts of distilled water (15% and 20%) and calcined OBFA (OBFA/c) (10%, 20%, 30%, and 40%) were used to prepare a group of samples. It was determined that mechanical and physical tests produced the best results for the 20% distilled water bricks. Indeed, at low water percentages, OBFA cannot dissolve properly, however, at higher values of biomass fly ash, a better dissolution could be observed due to the polymerization reaction improvement. Additionally, samples made with 30% OBFA/c and 20% distilled water demonstrate promising physical and mechanical qualities, with a compressive strength of 58.98 Mpa. However, a decrease in compressive strength was noted when OBFA incorporation exceeded 30% by weight. In the same study, Terrones-Saeta [90] investigated the feasibility of producing geopolymer using alpeorujo (BBA) (a by-product of oil composed of solid parts of olives and vegetable fats) as an alkaline activator and biomass clinker from the combustion of almond shells and Chamotte (C) as a source of aluminosilicate. The physical and aesthetic qualities of this geopolymer are comparable to those of conventional bricks. The compressive strength reaches a maximum value of 59.2 MPa for the ideal mixture of 40% chamotte (C) and 60% BBA.
Table 4. The chemical make-up of various lignocellulosic biomass ashes used in the production of unfired clay bricks.
| Oxide Cotenant | SBA [77] |
SCBA [80] |
RHA [83] |
BA [81] |
RHA [79] |
BBA [90] |
BBA [91] |
OPFA [88] |
OBFA/C [89] |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 35.17 | 51.66 | 89.08 | 35.17 | 93.15 | 11.21 | 46.10 | 1.86 | 56.35 |
| Al2O3 | 0.28 | 9.92 | 1.75 | 0.28 | 0.21 | 2.57 | 12.04 | 0.38 | 14.96 |
| FeO3 | 5.22 | 2.32 | 0.78 | 5.22 | 0.21 | 1.33 | 4.78 | 0.67 | 6.07 |
| CaO | 2.07 | 2.59 | 1.29 | 2.07 | 0.41 | 11.10 | 19.65 | 5.33 | 9.15 |
| MgO | 0.91 | 1.44 | 0.64 | 0.91 | 0.45 | 4.21 | 3.71 | 0.81 | 3.05 |
| Na2O | 0.01 | - | 0.85 | 0.01 | - | 0.22 | 0.78 | 0.19 | 0.59 |
| K2O | 3.75 | 2.10 | 1.38 | 3.75 | 22.31 | 23.91 | 4.59 | 52.1 | 4.52 |
| TiO2 | 0.02 | 0.74 | - | - | - | 0.12 | 0.83 | 0.05 | 0.74 |
| P2O5 | 1.05 | 0.90 | 0.62 | - | - | 3.58 | 1.12 | 1.62 | 0.15 |
Figure 9 presents a summary of the results of the compressive strength of various investigations of the geopolymer bricks.
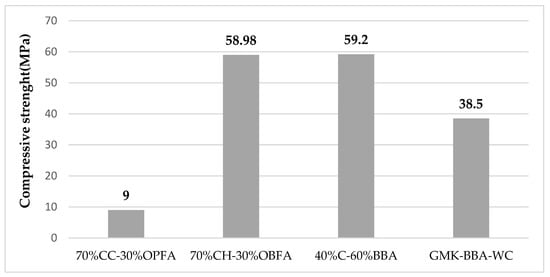
It is not possible to make a comparison in these studies. In fact, several factors, including the amount of reactive phases, the chemical makeup and types of aluminosilicate sources, the nature and concentration of the alkali silicate, the curing regimes, the fillers or additive content, as well as the water content, all have an impact on the formation of geopolymer bricks.
-
The influence of the clay calcination temperatureTo increase the reactivity of the clay used in the geopolymerization reaction, the clay must be calcined, which is a crucial step in the production of geopolymer bricks. The heat treatment transforms the crystalline phases of the clay into amorphous phases more reactive which contributes to the geoplymerization reaction and determines the final properties of the geopolymers. The optimal activation temperature depends on the mineralogical composition of the material. Thermogravimetric analysis (TGA) is commonly used to determine this ideal calcination temperature. The activation temperature must be at a temperature above the dehydroxylation peak observed on the derivative of the TGA curve or the differential thermal analysis (DTA) curve showing a downward pointing peak during dehydroxylation (endothermic reaction). One must determine the temperature that indicates the end of the peak on the DTG or DTA curve associated with the loss of hydroxyls from the used clay and before the peak showing the structural reorganization of the mineral during recrystallization. Indeed, at very high temperatures, some crystalline minerals can appear, which decreases the quantity of the amorphous phase in the precursor and thus its geopolymerization reactivity [92].
-
The influence of the activating solutionThe activating solution is an essential element in the geopolymerization process. Depending on its concentration and quantity, it will offer the right mixture to start the reaction and establish the final composition of the cured material. Additionally, it serves to speed up the breakdown of the aluminosilicate source, encouraging the development of stable gels with low solubility and the creation of compact structures using these gels. The most widely used activators are hydroxides or alkali silicates, specifically sodium hydroxide (NaOH) and potassium hydroxide (KOH). Sodium hydroxide solution is known to produce a better dissolution of the amorphous phase of aluminosilicates in combination with silicate monomers. On the other hand, potassium hydroxide solution is known to increase gel growth by association with larger aluminosilicate anions. Furthermore, the various studies conducted so far have proven that the presence of an alkali silicate solution leads to better microstructure and strength properties [93]. However, the cost of using silicate solutions during the geoplymerization reaction is high, not to mention the negative effects they have on the environment. These are prepared by dissolving glass that forms at a high temperature, which results in considerable greenhouse gas emissions [94]. It would be advantageous to use NaOH or KOH in place of the silicate solution.
-
The influence of the concentration of the alkaline solutionGenerally, the dissolution of aluminosilicate sources increases with the increase of the alkaline solution concentration, thus increasing the mechanical strength of the elaborated bricks. However, the compressive strength of geopolymer materials typically declines after a certain concentration. When using high concentrations, the activation of the clay requires an excess of hydroxide ions, which can be the cause of this decrease. Indeed, this excess caused the precipitation of the aluminosilicate gel at a very early stage. As a consequence, the geopolymerization was blocked, which led to geopolymers with low mechanical properties [95].
-
The influence of compaction parameterThe mechanical performance of geopolymer materials is directly influenced by the molding and compaction process. A significant improvement in compressive strength has been noted between geopolymer bricks prepared by applying compaction pressure during the molding process and those prepared without any compression during the molding process (vibration in the vibrating table or the impact table). For the study of [88], geopolymer brick molds were prepared using 70% calcined clays (CC) of Bailen and 30% by weight of olive pomace fly ash (OPFA) as a source of aluminosilicate and a solution of potassium silicate (KOH-K2SiO3) as an alkaline precursor, giving 60 blows in a shock table. Compressive strength of 9 MPa was observed. Nevertheless, for the study of [89], a strength of 59.2 MPa was determined for geopolymer bricks prepared entirely from chamotte, the residue of the ceramic industry as a source of aluminosilicate, and potassium oxide (KOH) contained in olive biomass fly ash as an alkaline solution, a compression of 50 MPa was used to mold specimens of an internal dimension of 6 × 3 cm. However, to achieve the 50 MPa for bricks with actual dimensions of 22 × 10.5 × 5 cm3, 1155 KN of force must be applied, which is completely unsuitable for use on an industrial scale.
2. Unfired Bricks: Advantages and Drawbacks
In comparison to fired bricks, stabilized unfired bricks permits the incorporation of higher levels of lignocellulosic biomass ash waste than fired bricks. The majority of unfired bricks are stabilized by the use of cementitious binders (lime/cement), but the high carbon footprint associated with the use of these binders is considered the main drawback. Geopolymer bricks were recently developed in the ceramic industry and were considered viable alternatives to cement-lime stabilized bricks. The stabilization of bricks by chemical binders using alkaline activation fits perfectly in the sustainable development context where a high volume of waste such as lignocellulosic biomass ashes could be reused and valorized in the clay matrix with a carbon footprint considered as zero. Indeed, as geopolymers have molecular structures close to zeolite, they can also immobilize toxic wastes or heavy metals contained in these ashes, thus decreasing the cost and the problem of burying this type of material. Nevertheless, different drawbacks limit the use of these geopolymer bricks on an industrial scale. Indeed, the alkaline activation of calcined clay is the most classical way and the most used in research to obtain a geopolymer with good mechanical properties and durability. Indeed, the calcination of clay allows for to modification of its crystalline structure into an amorphous structure and then improves its reactivity in an alkaline medium. Alkaline activation of clay soils at low temperatures is being researched more and more as a method of soil stabilization. Indeed, the use of low-quality raw clays other than kaolin and without thermal pretreatments, such as Montmorillonite, Illite, etc. would be a solution to reduce greenhouse gas emissions, manufacturing costs, and excessive use of kaolin by valorizing widely available natural clays at low-cost. This substitution would allow an energetic gain by overcoming the step of thermal treatment of clay. However, the understanding of the reactivity of soils using alkali activation stabilization is complex because of the different parameters that can influence their reactivity such as the clay composition of the soil, and the particle sizes, … In this context, a better understanding of alkaline activation of clay minerals without prior calcination is needed. The commercialization of these geopolymer bricks may also be constrained by the high cost and negative environmental impact associated with the use of alkaline activators in various research projects. The use of mixed binders (chemical and cementitious) seems to be appropriate to overcome the cost considerations for chemical binders. However, the carbon footprint of using cementitious binders is still there [96]. As an alternative to commercial alkaline activator solutions, research is focused on the use of lignocellulosic biomass ash, primarily olive waste rich in potassium oxide. These ashes are inexpensive, environmentally friendly, and can be used to create long-lasting, unfired clay bricks with good mechanical and physical qualities. These geopolymer bricks based on lignocellulosic ashes could compete with fired bricks which are preferred by their high mechanical resistance.
This entry is adapted from the peer-reviewed paper 10.3390/app122010669
This entry is offline, you can click here to edit this entry!
