Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, General & Internal
Microbiome studies have already demonstrated unique microbial patterns in systemic autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis, and systemic lupus erythematosus. Dysbiosis also seems to be associated with allergies, in particular asthma, atopic dermatitis, and food allergy.
- vitamin D
- microbiota
- immune system
- allergies
1. Introduction
In the last decade, a lot of studies have been conducted on the effects of both the microbiome and vitamin D on the immune system. Research indicates that the microbiota exhibits both pro-inflammatory and anti-inflammatory properties in direct or indirect ways [1]. It is now clear that alterations of the normal composition of the microbiome are narrowly associated with immunological disorders [2]. Vitamin D is a fat-soluble vitamin and a critical regulator of calcium and phosphate homeostasis and bone health [3]. Among other systemic effects, vitamin D, mainly through the vitamin D receptor (VDR), also has an important role in the modulation of immune response [4,5]. Vitamin D and the microbiome seem to affect the immune system in various yet similar ways, but is this due to an interaction and/or a synergistic effect between them? As a matter of fact, interactions between vitamin D, gut bacteria, and the immune system can occur at several levels and may include both the innate and the adaptive immune system.
2. Microbiome and Microbiota
The human microbiota is the community of commensal, symbiotic, and pathogenic microorganisms that survive on our body, skin, and respiratory, gastrointestinal and urogenital systems [6]. The composition of the microbiota is already formed in the early years of life but is dynamic and shaped by both genetic and non-genetic factors [2]. The microbiome is the set of genomes of our microbial symbionts [7]. Thanks to the advancement of technology, the genes of the microbial communities that colonize our organism have been sequenced over the last 10 years. The largest datasets reported come from the gut. The human microbiota consists of 12 different bacterial phyla, with 93.5% classified as Bacteroidetes, Proteobacteria, Firmicutes, Actinobacteria, or Euryarchaeota phyla [6]. Pathogenic microorganisms can be distinguished from commensal microbiota because they carry specific adhesive and invasive molecules, such as adhesins and invasine, which allow them to adhere and invade tissues, causing damage to the host. Early microbial colonization of the mucous membranes, such as the respiratory system and skin, occurs in tandem with the development of the immune system. During early microbial assembly, the immune system is susceptible to the colonization of organisms due to its immaturity: The decreased secretion of cytokines results in muted inflammatory responses, which allows the settlement and expansion of the microbiome in the various niches [8]. Germ-free mice provided key evidence on the importance of the microbiota to health, as these animals showed various immune defects and increased susceptibility to infections [1].
Figure 1 describes the regulatory effects of microbial exposure on Th2 cells.
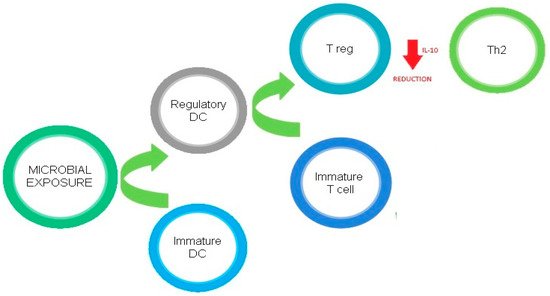
Figure 1. Regulatory effects of microbial exposure on Th2 cells.
3. Vitamin D
3.1. Synthesis and Metabolism
Vitamin D encompasses both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) [14]. In humans, the major source of vitamin D (90%) is the exposure to solar UVB radiation, which determines the formation of cholecalciferol in the skin, which is then metabolized in the liver, by the vitamin D 25-hydroxylase Cyp2R1 and to a lesser extent by Cyp27A1, to 25-hydroxyvitamin D (25-OH-D3) and finally carried to the kidneys, where it is transformed into the active form (1,25-dihydroxyvitamin D, 1,25-(OH)2D) [3]. Only 10% of vitamin D is obtained through food ingestion (with vitamin D-rich foods such as cod liver oil, tuna, sardines, milk, eggs, certain mushrooms, and fortified orange juice and dairy products) [15].
3.2. Mechanism of Action
l,25(OH)2D3 acts primarily through vitamin D receptors (VDR) [16]. VDR is a nuclear hormone receptor and transcription factor expressed in a variety of tissues, including the intestines, adipose tissue, and liver, as well as most immune cells, and modulates metabolic and immune system processes [17]. VDR has a key role in the modulation of the immune response since it is expressed in immune cells, including CD4+ and CD8+ T cells, B cells, neutrophils, and antigen-presenting cells (APCs) [4]. Many of these cells, such as macrophages and dendritic cells, are capable of synthesizing biologically active vitamin D from circulating 25OHD, which enables the rapid increase of local levels of vitamin D, potentially needed to shape adaptive immune responses [18]. VDR is also highly present in the small intestine and colon, where it plays critical roles in proliferation, differentiation, permeability, host–microbial interactions, immunity, and susceptibility to pathogenic infection. Notably, it is crucial for maintaining a healthy microbiome [19].
Figure 2 describes the effects of the VD/VDR complex on the immune system.
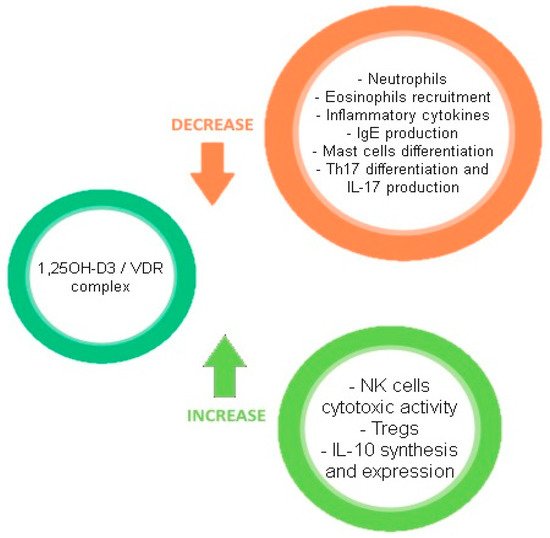
Figure 2. Effects of the VD/VDR complex on the immune system.
3.3. Systemic Effects and Deficiency
Vitamin D is an important regulator of the immune system, acting directly on immune cells to promote an anti-inflammatory state, and the balance between proinflammatory and anti-inflammatory activity is disrupted in vitamin D deficiency in favor of the former [6]. Populations with lower levels of vitamin D (i.e., those living furthest from the equator and those in early infancy) are more likely to develop several immune-mediated diseases, including allergic asthma and allergies to foods [14,20]. There is solid evidence that vitamin D supplementation can reduce the rate of infection, preventing autoimmune disorders, and there is promising data linking vitamin D deficiency to increased rates of childhood asthma and other allergic conditions [21]. Despite this, what represents adequate levels of vitamin D in the blood for human health generally and specifically for each of the reviewed allergic conditions remains controversial, as some observational studies seemed to confirm that vitamin D deficiency may contribute to increasing the risk of allergy and asthma. The necessity for further studies in this field is evident [18,22].
4. Linking Vitamin D, the Microbiome, and the Immune System
Both vitamin D deficiency and dysbiosis have been shown to impact systemic and chronic inflammation and to increase the risk of various conditions, including cardiovascular, neurological, infectious (including COVID-19), and metabolic diseases, autoimmune disorders, and cancer [4,5,23,24]. Focusing on allergies, their pathogenesis can be explained by both the hygiene hypothesis and the vitamin D hypothesis. The hygiene hypothesis was first proposed in 1989 by David P. Strachan [25], who postulated that infections in early childhood, transmitted by unhygienic contact with older siblings or acquired prenatally from the mother, could prevent the development of allergic diseases. An evolution of this theory is the old friend hypothesis. According to Rook [26], a hygienic lifestyle and cleanliness can be defined as an abuse of antibiotics, antibacterial soaps, and cleaners; delayed exposure to viruses; and an excessive time spent indoors. All of these can decrease immune tolerance and deplete indigenous commensal bacteria (the “old friends”). According to the vitamin D hypothesis, adequate vitamin D levels and supplementation in the first year of life can sensitize children against allergens and reduce the risk of development of food allergies and asthma [27]. On the other hand, high levels of vitamin D may also increase the risk of allergic sensitization by inhibiting the maturation of dendritic cells and the development of T-helper 1 responses [28]. Research into the gut microbiome and vitamin D is therefore considered to be promising for the understanding, treatment, and prevention of autoimmune and allergic diseases [29].
The effects of hypovitaminosis D and dysbiosis on immune system are depicted in Figure 3.
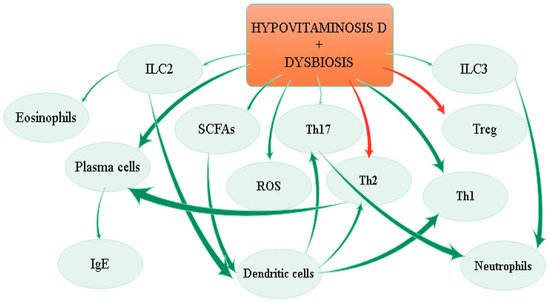
Figure 3. Effects of hypovitaminosis D and dysbiosis on the immune system.
5. Focus on Specific Allergies
5.1. Asthma
Asthma is a respiratory condition that likely results from complex interactions between multiple environmental and genetic influences. Proposed risk factors for asthma vary with the age of asthma onset and timing of exposures and behaviors relative to the onset of asthma. Microbes have long been postulated to play a role in asthma and might also shape its heterogeneity [73]. The early life microbiome likely influences the likelihood that an allergic predisposition results in asthma. Exposure to bacteria and bacterial products may influence the development of allergen sensitization and asthma, although the exact effects appear to depend on a complex interplay of timing of exposure (first year of life versus later in life), location, abundance, and diversity of the microbiome, and specific microbial products [74]. As an example, early life exposure to allergens and certain bacteria in the environment may lower the risk of asthma [75], whereas later life exposure to bacteria may increase the risk of asthma [76]. The mechanism for this protective effect is not known, but changes in gut microflora and related effects on innate immunity are hypothesized. Moreover, studies have suggested that interactions between RSV and nasopharyngeal microbiota might modulate the host immune response, potentially affecting clinical disease severity such as the risk of wheezing and symptoms of asthma. Respiratory virus infection has been shown to alter the airway microbiome, with increased detection of Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae in children with and without asthma [77]. This coalescing of antimicrobial immune responses was associated with increased respiratory symptoms. Since most asthma has its origins in childhood, early nutrition, including prenatal exposure to nutrients, may be relevant as a risk factor for the development of asthma and allergies [78]. It remains unclear whether vitamin D supplementation has a role in asthma prevention. There are conflicting reports on the association between vitamin D status and allergic diseases. In some reports, vitamin D deficiency (in pregnant women, children, or adolescents) was associated with increased as well as with decreased frequency of allergic diseases such as asthma or eczema [79,80]. The Vitamin D Antenatal Asthma Reduction Trial (VDAART) attempted to answer the question of whether vitamin D supplementation to women during pregnancy can prevent the development of asthma and allergies in their children. The aim of this study was to determine whether children born to mothers who had received 4400 IU of vitamin D3 per day during pregnancy (vitamin D group) would have a lower incidence of asthma and recurrent wheeze at the age of six years than would those born to mothers who had received 400 IU of vitamin D3 per day (control group). The six-year follow up showed that the prenatal period alone did not influence the incidence of asthma and recurrent wheeze among children who were at risk for asthma [81,82]. Randomized trials examining the effect of vitamin D supplementation on asthma outcomes were inconclusive. Larger trials with longer-term follow-up are needed.
5.2. Atopic Dermatitis
A multiplicity of factors, including skin barrier abnormalities, defects in innate immunity response, Th2-skewed adaptive immune response, and altered skin resident microbial flora are involved in the pathogenesis of atopic dermatitis [83]. The increased prevalence of AD, particularly in industrialized regions, has been hypothesized to be due to excessive hygiene accompanying the Western lifestyle, reducing exposure of the host’s immune system to education provided by beneficial microbes. A subset of patients with AD are prone to microbial dysbiosis with bacteria, viruses, and fungi, which can exacerbate skin inflammation [84]. For example, Staphylococcus aureus is the most common skin-cultured pathogen of AD and its colonization is associated with increased IgE responses, food allergy, and AD skin-disease severity [85,86]. There is increasing evidence that commensal skin microbes from normal skin can improve the skin barrier and augment host defense against skin pathogens, including S. aureus. Staphylococcus epidermidis and Staphylococcus hominis secrete antimicrobial activities that inhibit S. aureus growth and biofilm formation [87,88]. S. epidermidis has also been found to stimulate Toll-like receptor 2 to induce production of keratinocyte-derived antimicrobial peptides and increase tight junctions to enhance the skin barrier [89]. These protective strains are deficient in atopic dermatitis [85]. Using antibiotics in the treatment of S. aureus infection is disadvantageous because they kill not only S. aureus, but also beneficial bacteria and potentially select antibiotic-resistant bacteria such as methicillin-resistant S. aureus, underlining the importance of maintaining the skin-resident normal microbial flora. A few small, randomized trials have evaluated the role of vitamin D supplementation in the prevention of winter-related exacerbation of atopic dermatitis [90,91,92]. In the largest study, 107 children with a history of atopic dermatitis worsening during winter were treated daily with 1000 international units of vitamin D or placebo for one month. The primary outcome was a reduction in the clinician-measured eczema area and severity index (EASI). At the end of the study, the mean decrease in the EASI score was 6.5 in the vitamin D group and 3.3 in the placebo group [90]. Although the results of these trials suggest that winter supplementation of vitamin D may be beneficial for patients with atopic dermatitis, larger, well-designed studies are needed to clarify the role of vitamin D in the prevention and treatment of atopic dermatitis.
5.3. Food Allergy
Tolerance is the normal immune response to the food an individual eats over a lifetime [93]. Food allergy is thought to involve deviation from the default state of mucosal immune tolerance that can be driven by diet, commensal microbiota, and the interactions between them [94]. Although alterations in the intestinal microbiome are known to be associated with the development of asthma, less is known regarding the role of microbiome alterations in food allergy development. In their prospective microbiome-wide association study of food sensitization and food allergy in early childhood, Savage et al. [95] collected intestinal microbiome samples at age three–six months in children participating in the follow-up phase of VDAART. As a result, the study suggested that the microbiome may have a causal role in the development of food allergy. Specific patterns of microbiota colonization, such as colonization in large numbers by probiotics or greater microbial diversity, may favor tolerance, possibly through increased production of IgA and IL-10 [96]. It is postulated that cesarean delivery compared to natural childbirth increases the risk of IgE-mediated sensitization to food allergens as a result of alterations in the gut microbiota [97]. Data on the impact of cesarean delivery on the rate of clinical food allergy are inconsistent, although most studies show an increased risk [98,99]. Studies of children with a milk allergy have shown that infants with a milk allergy have higher total bacteria and anaerobic counts compared to healthy control subjects [100]. Reduced microbial richness and increased Bacteroides were observed in subjects with a self-reported peanut or tree nut allergy compared to those not reporting these allergies [101]. Murine models of food allergy have provided experimental dimensions to the study of the microbiota and food allergy. In a mouse model of induced food allergy by skin sensitization, feeding mice a high-fat diet prior to skin sensitization and subsequent gastrointestinal challenge to a food allergen was associated with the development of obesity, decreased intestinal bacterial diversity, and increased food allergy susceptibility [102]. Transfer of the gut microbiome from these mice to germ-free recipient mice on a normal diet led to decreased intestinal bacterial diversity and increased food allergy susceptibility, but not obesity. Translation of these observations to humans requires further study. Some murine studies have shown that Clostridia strains in particular modulate allergy. Imbalances in T helper cell subsets (Th1/Th2) in cellular immunity contribute to the development of food allergy. Seventeen strains of bacteria from human stool that enhance regulatory T-cell abundance have been identified. Oral administration of these 17 Clostridia strains into mice attenuated disease in models of colitis and allergic diarrhea [103]. Therapy with a consortium of protective clostridial species suppresses food allergy in mice [104], suggesting that gut microbiota dysbiosis is a potential target for future treatment of food allergy if these findings are replicated in humans. Similarly, skin commensal bacteria have been recognized as significant factors imprinting the immune system [105], possibly also influencing food allergy. Skin colonization with S. aureus, a marker for more severe eczema, is also associated with sensitization to food allergens [85]. Independent of eczema severity, skin S. aureus colonization was associated with hen′s egg and peanut sensitization and persistent allergy, with a weaker association seen for cow′s milk, in a study that followed children from infancy to six years of age [106]. As a matter of fact, although strict allergen avoidance is still the key treatment for food allergy, there is a greater focus on the preventive effect of the addition of lactose and probiotics to hypoallergenic infant formulas in order to modulate the gut microbiome and early immune responses in high-risk populations, either in families with a history of allergies, or in infants who are showing evidence of food sensitization or eczema [28,107]. Only a few trials of probiotics for the prevention or treatment of challenge-proved food allergies have been published. Trials of probiotic supplementation with Lactobacillus casei and Bifidobacterium lactis for 12 months showed no effect on milk allergy resolution, although Lactobacillus rhamnosus combined with extensively hydrolyzed casein formula increased rates of milk allergy resolution compared to a control group receiving formula alone [108,109]. The probiotic Lactobacillus rhamnosus GG administered with peanut oral immunotherapy for 18 months induced desensitization compared to a placebo. However, because there was no oral immunotherapy-only or probiotic-only group, the efficacy of the probiotic itself is unclear. The effects of probiotic treatment are likely strain specific, and the data are currently inconclusive to support probiotic supplementation with specific taxa for food allergy [110]. Early infancy could be the key window of opportunity for intervention given age-dependent associations between the gut microbiome and food allergy outcomes. Gut microbial richness at the age of three months is associated with an increased likelihood of food sensitization by the age of one year [111]. Murine models also support age-sensitive interactions with microbiota [112]. Colonization of germ-free mice with a diverse microbial population early but not late in life suppresses IgE and prevents mice from having a food allergy [113]. These collective findings support the notion that microbial effects on early immune system development play a role in subsequent food allergy development. Epidemiologic studies suggest possible associations between vitamin D deficiency and a variety of conditions, but a causal relationship has not been established. These include certain immunologic conditions such as food allergies and asthma in adolescents [114]. Vitamin D has a potentially significant role for improving the symptoms and severity of food allergy for its immunomodulatory effects. Proposed but unproven theories suggest a role for both vitamin D excess and deficiency in the development of food allergy. Future studies for assessing adequate amounts of vitamin D intake for the treatment and prevention of food allergy are also warranted.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22084288
This entry is offline, you can click here to edit this entry!
