Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Benzimidazole is an important heterocyclic fragment, present in many biologically active compounds with a great variety of therapeutic purposes. Most of the benzimidazole activities are explained through the existence of 1,3-tautomeric equilibrium. As the binding affinity of each tautomer to a protein target depends on an established bioactive conformation, the effect of tautomers on the ligand protein binding mechanism is determinant.
- benzimidazoles
- 13C NMR
- tautomeric equilibrium
- mesomery
1. Introduction
The tautomerism phenomenon is considered as a dynamic equilibrium between interconvertible structural isomers, named tautomers, with the migration of one atom or group. When a hydrogen atom migrates, the phenomenon is known as prototropy; however, other groups such as alkyl, aryl, acyl, cyano, halogens, amines, and nitro, as well as methals can migrate; Scheme 1 [1].
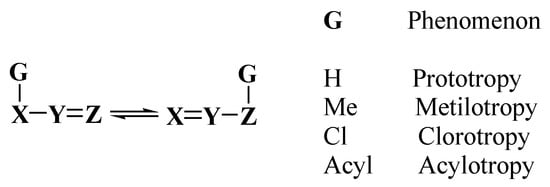
Scheme 1. Tautomeric equilibria in organic compounds.
Nuclear magnetic resonance (NMR) spectroscopy is one of the most useful techniques to study tautomerism. The signals observed in the spectra depend on the activation energy between the tautomers, which determines their lifetimes and chemical shift difference (Δδ). When lifetimes are long, compared with 1/Δδ, a slow exchange regime gives rise to separate narrow signals for each of the tautomers. In this case, integration of the 1H-NMR signal intensities is the method of choice to study the tautomerism. For shorter lifetimes, the exchange is raised, leading to line broadening. Then, high magnetic fields and lower temperatures can be used to achieve slow exchange conditions. Interpolation is the method of choice when lifetimes are shorter than 1/Δδ, and fast exchange and signal coalescence predominate to give averaged narrow signals, but the NMR chemical shifts of the individual tautomers remain unknown. Four techniques have been used to solve this problem: (1) the use of blocked derivatives of individual tautomer, replacing the tautomeric proton by a methyl group, and performing a correction for the substituent effect; (2) the use of model compounds that exclusively exist in one tautomeric form; (3) the use of the properties measured in the solid state where only one tautomer exists, but phase effects should be considered; and (4) the use of theoretically calculated properties as GIAO absolute shielding, however, solvent effects are difficult to estimate.
Benzazoles (BZs) are constituted by a benzene ring fused to an oxazole (BO), thiazole (BT), or imidazole (BI) ring; left in Figure 1. A typical kind of tautomerism in BI is the relocation of a proton, known as annular tautomerism (a). BZs containing an exocyclic heteroatom, where a proton can migrate from cyclic nitrogen to an exocyclic heteroatom, retaining the aromaticity, represent an exocylic tautomerism (b); right in Figure 1.
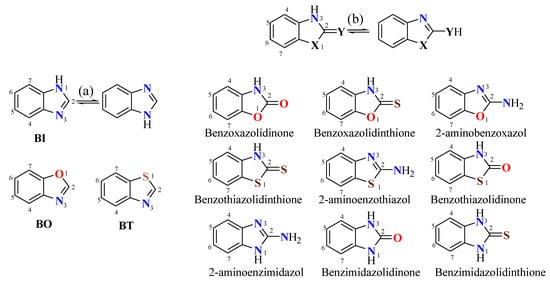
Figure 1. (a) Intracyclic and (b) exocyclic tautomerism in benzazoles (BZs).
In commercial databases, the presence of tautomeric duplicates has been found, and both tautomers are offered as different products [2]. On the other hand, different names for tautomers based on the IUPAC rules have been used [3]. In general, compounds with well-known tautomerism are named as the predominant tautomer at equilibrium. For example, 2-mercaptobenzimidazole (MBI) is named as benzimidazolidinethione (BIT) or 1,3-dihydro-2H-benzo[d]imidazole-2-thione (X = NH, Y = S); Figure 1.
The tautomeric interconversion with a low-energy barrier, in general, displays averaged signals in the NMR spectrum. To slow down the interconversion rate in solution, the following strategies are used: lowering temperature and/or the use of hexamethylphosphoramide-d18, which prevents the formation of hydrogen bonds. Thermodynamic and kinetic aspects of the tautomeric equilibrium have been explored using dynamic NMR studies [4][5][6] Because of the wide range of chemical shifts and high sensitivity, 15N-NMR spectroscopy has been used to study the tautomerism of nitrogen in heteroaromatic compounds [7]. Solid-state NMR experiments [4] 4, X-ray crystallography [8][9][10], and 15N-NMR studies in DMSOd6 for both BIT and 1-MeBIT revealed that the thione form is the predominant tautomer [11]. Recently, Pandey et al. theoretically studied 5-MeOBIT using B3LYP methods with a 6-311++G (d, p) basis set. A comparison between the experimental and calculated structure as well as the calculated and experimental 1H and 13C chemical shifts showed a good correlation with the thione isomer [12].
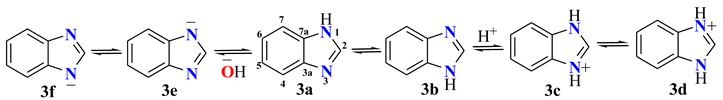
Among the BZs, benzimidazole (BI) is the most known and the one whose tautomeric equilibria have been widely studied [4][5][6][7]. It has been stablished that, when a large substituent is bonded at the N1 position in 2-heterosubstituted BIs, two isomeric compounds exist because of the annular tautomerism. BI is an important heterocycle, present in several biologically active compounds. It has been found in progesterone receptor antagonists [13], luteinizing hormone-releasing hormone antagonists (leuprolide, goserelin, triptorelin) [14][15], antiviral (enviradine) [16][17], antiprotozoal [18][19], antimicrobial [20][21][22], analgesic and anti-inflammatory [23], anticonvulsant, antidiabetics [24], anthelmintics (albendazole, mebendazole, and thiabendazole), proton pump inhibitors (omeprazole, lansoprazole, and pantoprazole), antihistaminic (astemizole), and antihypertensives (candesartan, cilexitil, and telmisartan), among others.
The binding affinity of each tautomer to a protein target depends on an established bioactive conformation. Therefore, the method proposed herein to calculate in solution the prototropic ratio in BZs, on the basis of the electronic effect of pyrrole like atom (NH = Npr) and pyridine like atom (Npd) on C4 and C7 chemical shift resonances, is very valuable. On the other hand, the method is also helpful to correctly assign the carbon atoms on the benzene ring.
2. Estimation of the Anular Tautomerism on Benzimidazol
The chemical shift reference values for the estimation of the tautomeric equilibrium were set using the 1-methyl-benzimidazole (1-MeBI, 4) as a model compound. In case 4, the tautomeric equilibrium is non-existent; therefore, a set of seven narrow signals are observed in CDCl3. The δC4 appears at 120.4 ppm, characteristic of an Npd effect, whereas the δC7 at 109.5 ppm is characteristic of an Npr effect. In DMSOd6, the δC4 and δC7 values are 119.2 and 110.1, respectively. Then, a good approximation for 100% of the pyridine like character of δC4 and 100% of the pyrrole like character of δC7 is 120.0 and 110.0 ppm, respectively. Furthermore, the tautomeric proportion in BIs can be calculated on a base equal to 120.0 ppm for δC4ref (Npd) and 110.0 ppm for δC7ref (Npr) as reference values (Figure 2).
The tautomerism in BIs can be calculated considering that the observed chemical shift δobsC4/C7 is the result of the averaged C4 and C7 chemical shifts weighted by the respective molar fraction contributions xpd (pyridine character) and xpr (pyrrolic character). Equations (1)–(3) can be used to calculate the molar fractions of each tautomer; Figure 2.
δobs = (xpdδC4ref) + (xprδC7ref)
xpd= (δobs − δC7ref)/(δC4ref − δC7ref)
xpr = 1 − xpd

Figure 2. Effect of heteroatom X on C7 and C5 in 1,3-benzoheterazoles 1–3.
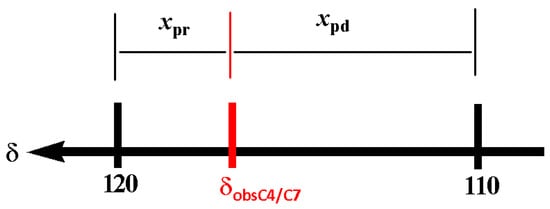
Figure 3. 13C chemical shifts as references to calculate the tautomeric proportion of pyrrole–pyridine nitrogen atoms.
From the reported 13C data for BI 3, as well as their protonated and deprotonated derivatives, in DMSOd6 as solvent, Table 1 [25], the tautomeric proportion can be calculated. For BI, the δobs value is 116.3, then the estimation is 63% of the pyridine character (+N3H). In the case of δobs for protonated BI (118.3 ppm), a small effect of positive charge on nitrogen atoms shifts this signal to high frequencies in approximately 2.0 ppm. On the contrary, any effect of the negative charge on nitrogen in deprotonated BI was observed.
Table 1. Protonation and deprotonation of BMZ 13C in DMSOd6.
| Comp. | C2 | C3a | C4 | C5 | C6 | C7 | C7a |
|---|---|---|---|---|---|---|---|
| 3a/3b | 142.5 | 139.0 | 116.3 | 122.3 | 122.3 | 116.3 | 139.0 |
| [3c/3d]+ | 143.6 | 133.8 | 118.3 | 130.0 | 130.0 | 118.3 | 133.8 |
| [3d/3f]− Na+ | 153.1 | 146.9 | 116.4 | 116.7 | 116.7 | 116.4 | 146.9 |
| [3d/3f]− Li+ | 153.5 | 147.0 | 116.8 | 117.0 | 117.0 | 116.8 | 147.0 |
This approximation can be applied to 2-aminomethylbenzimidazole (2-AMBI); Scheme 2. In the work of Sierra-Centeno et al. [26], the acid–base equilibrium constants of 2AMBI were determined in aqueous solutions at 25 °C through 13C NMR spectroscopy, potentiometric and spectrophotometric techniques, as well as through theoretical methods. 13C NMR spectra were recorded for solutions of 0.1 M of 2-AMBI at pH values from 1.1 to 13.2, containing 10 % v/v of D2O. During the titration process, only five signals were observed. The sets of C5 and C6 signals were shifted approximately 7.0 ppm to lower frequencies and C2 and C3a/C7a in 10.0 and 15.5 ppm, respectively, to higher frequencies, whereas the set of C4/C7 signals remained almost constant at 114.5 ppm. Therefore, the BI heterocycle retains its 45% pyridine character independently from the pH.
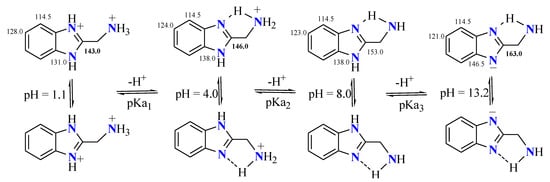
Scheme 2. Titration process of 2-(aminomethyl)benzimidazole dihydrochloride.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196268
References
- Elguero, J. Una Aproximación a la Tautomería de los Heterociclos Aromáticos. Rev. Soc. Quíim. Perú. 2020, 86, 452–476.
- Guasch, L.; Yapamudiyansel, W.; Peach, M.L.; Kelley, J.A.; Barchi, J.J.; Nicklaus, M.C. Experimental and Chemoinformatics Study of Tautomerism in a Database of Commercially Available Screening Samples. J. Chem. Inf. Model. 2016, 56, 2149–2161.
- Proj, M.; Sosič, I.; Gobec, S. Synthesis and NMR spectroscopic assignment of chlorinated benzimidazole-2-thione derivatives. Tetrahedron Lett. 2019, 60, 151078.
- Nieto, C.I.; Cabildo, P.; García, M.A.; Claramunt, R.M.; Alkorta, I.; Elguero, J. An experimental and theoretical NMR study of NH-benzimidazoles in solution and in the solid state: Proton transfer and tautomerism. Beilstein J. Org. Chem. 2014, 10, 1620–1629.
- García, M.A.; Claramunt, R.M.; Solcân, T.; Milata, V.; Alkorta, I.; Elguero, J. 13C and 15N NMR spectra of aminobenzimidazoles in solution and in the solid state. Magn. Reson. Chem. 2009, 47, 100–104.
- Claramunt, R.M.; López, C.; Alkorta, I.; Elguero, J.; Yang, R.; Schulman, S. The tautomerism of Omeprazole in solution: A 1H and 13C NMR study. Magn. Reson Chem. 2004, 42, 712–714.
- Larina, L.I. Chapter Five, Tautomerism and Structure of Azoles: Nuclear Magnetic Resonance Spectroscopy. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 233–321.
- Form, G.R.; Raper, E.S.; Downie, T.C. The Crystal and Molecular Structure of 2-Mercaptobenzimidazole. Acta Crystallogr. 1976, B32, 345–348.
- Khan, H.; Badshah, A.; Shaheen, F.; Gieck, C.; Qureshi, R.A. 1-Methyl-1H-benzimidazole-2(3H)-thione. Acta Crystallogr. Sect. E. Struct. Rep. Online 2008, 6, o1141.
- Palomo-Molina, J.; García-Báez, E.V.; Contreras, R.; Pineda-Urbina, K.; Ramos-Organillo, A. Aminosilanes derived from 1H-benzimidazole-2(3H)-thione. Acta Cryst. Struct. Chem. 2015, C71, 788–792.
- Balestrero, R.S.; Forkey, D.M.; Russell, J.G. Iminol-Thioamide Tautomerism of 2-mercaptobenzazoles and 1-methyl-2-mercaptoimidazoles. Magn. Reson. Chem. 1986, 24, 651–655.
- Pandey, M.; Muthu, S.; Gowda, N.N.M. Quantum mechanical and spectroscopic (FT-IR, FT-Raman,1H,13C NMR, UV-Vis) studies, NBO, NLO, HOMO, LUMO and Fukui function analysis of 5-Methoxy-1H-benzoimidazole-2(3H)-thione by DFT studies. J. Mol. Struct. 2017, 1130, 511–521.
- Zhang, P.; Terefenko, E.; Kern, J.; Fensome, A.; Trybulski, E.; Unwalla, R.; Wrobel, J.; Lockhead, S.; Zhu, Y.; Cohen, J.; et al. 5-(3-Cyclopentyl-2-thioxo-2,3-dihydro-1H-benzimidazol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile: A novel, highly potent, selective, and orally active non-steroidal progesterone receptor agonist. Bioorg. Med. Chem. 2007, 15, 6556–6564.
- Hashimoto, K.; Tatsuta, M.; Kataoka, M.; Yasoshima, K.; Shogase, Y.; Shimazaki, M.; Yura, T.; Li, Y.; Yamamoto, N.; Gupta, J.B.; et al. Benzimidazole derivatives as novel nonpeptide luteinizing hormone-releasing hormone (LHRH) antagonists. Part 1: Benzimidazole-5-sulfonamides. Bioorg. Med. Chem. Lett. 2005, 15, 799–803.
- Tatsuta, M.; Kataoka, M.; Yasoshima, K.; Sakakibara, S.; Shogase, Y.; Shimazaki, M.; Yura, T.; Li, Y.; Yamamoto, N.; Gupta, J.; et al. Benzimidazoles as non-peptide luteinizing hormone-releasing hormone (LHRH) antagonists. Part 3: Discovery of 1-(1H-benzimidazol-5-yl)-3-tert-butylurea derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 2265–2269.
- Gardiner, J.M.; Loyns, C.R. Synthesis of novel 1-, 1,4- and 1,7-substituted 2-mercapto- and 2-methylmercapto- benzimidazoles: Acyclic analogues of the HIV-1 RT inhibitor, TIBO. Tetrahedron 1995, 51, 11515–11530.
- Hwu, J.R.; Singha, R.; Hong, S.C.; Chang, Y.H.; Das, A.R.; Vliegen, I.; De Clercq, E.; Neyts, J. Synthesis of new benzimidazole–coumarin conjugates as anti-hepatitis C virus agents. Antivir. Res. 2008, 77, 157–162.
- Mavrova, A.T.; Vuchev, K.; Anichina, K.; Vassilev, N. Synthesis, antitrichinnellosis and antiprotozoal activity of some novel thieno pyrimidin-4(3H)-ones containing benzimidazole ring. Eur. J. Med. Chem. 2010, 45, 5856–5861.
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{sulfanyl}-1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 4221–4224.
- Desai, K.G.; Desai, K.R. Green route for the heterocyclization of 2-mercaptobenzimidazole into β-lactum segment derivatives containing –CONH– bridge with benzimidazole: Screening in vitro antimicrobial activity with various microorganisms. Bioorg. Med. Chem. 2006, 14, 8271–8279.
- Márquez-Navarro, A.; Nogueda-Torres, B.; Hernández-Campos, A.; Soria-Arteche, O.; Castillo, R.; Rodríguez-Morales, S.; Yépez-Mulia, L.; Hernández-Luis, F. Anthelmintic activity of benzimidazole derivatives against Toxocara canis second-stage larvae and Hymenolepis nana adults. Acta Trop. 2009, 109, 232–235.
- Kazimierczuk, Z.; Andrzejewska, M.; Kaustova, J.; Klimešova, V. Synthesis and antimycobacterial activity of 2-substituted halogenobenzimidazoles. Eur. J. Med. Chem. 2005, 40, 203–208.
- Kankala, S.; Kankala, R.K.; Gundepaka, P.; Thota, N.; Nerella, S.; Gangula, M.R.; Guguloth, H.; Kagga, M.; Vadde, R.; Vasam, C.S. Regioselective synthesis of isoxazole–mercaptobenzimidazole hybrids and their in vivo analgesic and anti-inflammatory activity studies. Bioorg. Med. Chem. Lett. 2013, 23, 1306–1309.
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759.
- Ceniceros-Gómez, A.E.; Ramos-Organillo, A.; Hernández-Díaz, J.; Nieto-Martínez, J.; Contreras, R.; Castillo-Blum, S.E. NMR Study of the Coordinating Behavior of 2,6-bis(Benzimidazol-2’-yl)pyridine. Heteroat. Chem. 2000, 11, 392–398.
- Sierra-Zenteno, A.; Galán-Vidal, C.A.; Tapia-Benavides, R. Acid-base equilibrium studies of 2-(aminomethyl)benzimidazole in aqueous solution. Rev. Soc. Quím. Méx. 2002, 46, 125–130.
This entry is offline, you can click here to edit this entry!
