Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A poor nutritional status such as malnutrition, unhealthy dietary behaviors, and a suboptimal dietary intake can play a significant role in the occurrence, development, and prognosis of chronic pain. The relationship between nutrition and chronic pain is complex and may involve many underlying mechanisms such as oxidative stress, inflammation, and glucose metabolism. As such, pain management requires a comprehensive and interdisciplinary approach that includes nutrition. Nutrition is the top modifiable lifestyle factor for chronic non-communicable diseases including chronic pain.
- diet
- nutrition
- nutrition recommendation
- chronic pain
1. Introduction
Chronic pain, as defined by The International Association for the Study of Pain (IASP), is pain that persists or recurs for more than 3 months [1]. Chronic pain is a serious health issue, affecting approximately 20% of adults worldwide and it is anticipated that this will continue to increase alongside the growing and ageing population [1]. There is also a significant socioeconomic burden associated with chronic pain, including high health care use and costs, high absenteeism, loss of productivity, functional impairment, and disability [2]. Due to the complexity of chronic pain and its comorbidities, both evidence and clinical practice have guided the development of integrative pain management, from monodisciplinary to multidisciplinary treatments and from multidisciplinary treatments to interdisciplinary programs, based on a biopsychosocial approach [3,4,5].
An accumulating body of evidence suggests that poor nutrition, such as malnutrition, unhealthy dietary behaviors, and a poor dietary intake can play a significant role in the occurrence, prognosis, and maintenance of chronic non-cancer pain, hereafter described as chronic pain [4,6,7]. Unhealthy dietary behaviors and a poor dietary intake is characterized by the limited intake of core nutrient-rich foods and an excessive intake of energy-dense nutrient poor foods [8]. The role of nutrition as an important lifestyle factor in pain management is gaining more attention. Over the past two decades, nutrition has occasionally been acknowledged by pain organizations, health care professionals, and consumers, and the interest in the role of nutrition in pain management has grown significantly. In a submission to the European Parliament in 2001, poor appetite and nutrition were listed, amongst others, as a burden associated with chronic pain [9]. In 2013, a qualitative study conducted by Chronic Pain Australia, an organization representing consumers experiencing pain, reported that individuals wanted more information on nutrition and pain management [10]. In 2015, Australia’s Faculty of Pain Management acknowledged that dietitians should provide input into patient care, where necessary [11]. Nutrition was also a major focus in the Consortium Pain Task Force White Paper, in 2018 [12]. More recently, in 2020, the IASP recognized the importance of optimizing one’s dietary intake in pain management strategies based on a large body of evidence, which indicated the significant effect of nutrition-based interventions on pain reduction [13,14].
2. The Nutrition-Related Health of People Experiencing Pain
2.1. Underweight, Overweight, and Obesity
Population-based studies suggest that there is a higher prevalence of chronic pain amongst people with an unhealthy weight (i.e., underweight, overweight, or obese), compared to those who are of a healthy weight [20,21,22]. Underweight is defined as a body weight below the healthy weight range, while overweight and obesity are defined as an excessive and abnormal increase in white adipose tissue. The body mass index (BMI) is a weight-for-height index (kg/m2), which is commonly used to classify the weight status in adults. A BMI of less than 18.5 kg/m2 is defined as underweight, between 25.0 kg/m2 and 29.9 kg/m2 is defined as overweight and over 30 kg/m2 is considered obese. Obesity also consists of three subclasses: class I (30–34.9 kg/m2), class II (35–39.9 kg/m2), and class III (≥40 kg/m2). There are limitations associated with the BMI as it does not take into account ethnicity or body composition such as fat and muscle mass. It also does not factor in the biological, physical, economic, psychological, and social aspects that contribute to weight status. Therefore, it is important to ensure that health professionals use a holistic approach to measuring health and do not rely on weight and the BMI alone.
Recent evidence has acknowledged the importance of nutritional factors affecting specific pain conditions. For example, studies exploring fibromyalgia have demonstrated that overweight or obese patients experienced more pain, impaired function, had higher levels of depression, and medication use than patients who were normal weight [51,52]. Underweight, overweight, and obesity coexist with chronic pain due to the nutrition-related underlying mechanisms. There is an interrelationship between the nutritional status, chronic pain pathophysiology, and eating behaviors. Diet profoundly impacts the body and has a complex relationship with the pain experience [4,13]. Dietary intervention (i.e., diet patterns and eating behaviors) has also been identified as one of the integrative treatments to alleviate chronic pain [4,53]. According to the existing evidence, common chronic pain conditions have been associated with nutritional factors, such as osteoarthritis [54], rheumatic arthritis [55], fibromyalgia [56], back pain [57], irritable bowel syndrome (IBS) [49], pelvic pain (e.g., endometriosis) [58], diabetic neuropathy [59], migraine headache [60], post-herpetic neuralgia [61], and carpal tunnel syndrome [62]. A summary of common pain locations related to over- and undernutrition are shown in Figure 1. Multiple site pain conditions and spreading pain conditions, such as myofascial pain syndrome and fibromyalgia are not illustrated in the figure. Based on the IASP classification of chronic pain [63], these pain conditions may not always belong to one category (nociceptive, nociplastic, or neuropathic pain), depending on the grading of the predominant central sensitization [64,65].
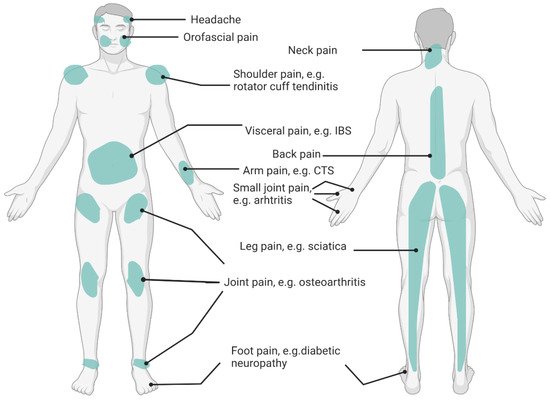
Figure 1. Pain Sites Related to Poor Nutrition. IBS: Irritable Bowel Syndrome; CTS: Carpal Tunnel Syndrome.
Poor nutrition not only impacts chronic pain pathophysiology, but also impacts other health outcome measures. For instance, compared with non-obese patients, obese patients with chronic pain had more physical limitations [66,67], a lower psychological wellbeing [68], more sleep disturbances [69,70], a poor health-related quality of life (HRQoL) [71], and a function dependence [72,73]. Multiple physical and/or mental diseases also frequently coexist with chronic pain, such as type 2 diabetes, cardiovascular disease or metabolic syndrome, anxiety (or post-traumatic stress syndrome), and depression [21,74]. These conditions can be modified using nutrition-related treatments.
2.2. Eating Behaviors and Dietary Preferences in People Experiencing Chronic Pain
Optimal dietary and nutrient intake are essential elements of musculoskeletal health. In addition to weight changes, a suboptimal nutrient intake and poor eating behaviors can cause altered serum nutrient levels, which can be observed among the patients with chronic pain. For instance, high levels of serum glutamate and aspartate were reported in patients with chronic migraine, orofacial pain, fibromyalgia, and complex regional pain syndrome [75,76,77,78]. Low levels of nutrients are also commonly recognized, such as vitamin D, omega-3 polyunsaturated fatty acid, vitamin B12, magnesium, zinc, ferritin, selenium, and folic acid [56,79]. Although, these studies do not draw conclusive and direct links with the aetiology of chronic pain, it is anticipated that chronic pain patients may have altered eating behaviors, either before the onset of pain or during the development of pain.
There is also an association between a suboptimal dietary intake and some pain conditions, such as irritable bowel syndrome (IBS) and pelvic pain syndromes [80,81]. Some special but diverse dietary triggers have been reported by headache patients (particularly migraines) [60]. It is also suggested that people experiencing pain generally consume more calories, added sugars, saturated fatty acids, sodium, and caffeine. This association has been demonstrated in a cross-sectional study that found one third of males and approximately half of female participants were consuming more than the recommended daily caloric intake, moderate fat intake, and a high saturated fat intake [82]. This study also showed that the intake of vitamin D, vitamin E, and magnesium, in people experiencing chronic pain, was lower than the recommended daily intake. Data from the British Birth Cohort Study has been analyzed and showed that women with chronic pain were more likely to decrease their intake of fruit and vegetables, and increase their high fatty foods consumption over time, compared to women without chronic pain [83]. The low intake of micronutrients has also been reported in another patient population with rheumatoid arthritis [84]. Another study observed that obese osteoarthritis patients had an increased calorie, fat, and sugar intake and this impacted on their pain severity [85]. Additionally, for patients with undernutrition, pain experiences could be accompanied by a loss of appetite and a decreased food intake [24,25,86,87]. This could lead to a poor dietary intake or absorption of nutrients (i.e., medications that affect gastrointestinal functions [87]) and subsequently a decreased fat free mass and impaired physical and mental functions (i.e., daily functioning and cognitive functions [86]).
3. The Underlying Potential Mechanisms That Explain the Interaction between Nutrition and Chronic Pain
The interaction between nutrition and chronic pain is bidirectional. However, it is not clear how nutritional factors interact with the pain generating mechanisms and the potential mechanisms that contribute to this relationship. Identifying and understanding these mechanisms can potentially increase the effectiveness of nutrition assessments and treatments in chronic pain management. The potential action mechanisms of the nutritional factors in chronic pain management have been identified and illustrated in Figure 2.
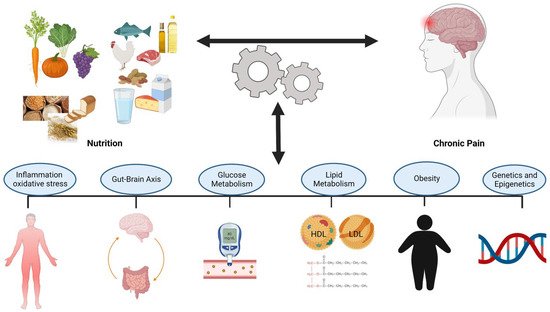
Figure 2. Potential Mechanisms of the Interaction Between Nutrition and Chronic Pain.
3.1. Inflammation and Oxidative Stress
Oxidative stress is defined as an increase in the reactive oxygen species produced as a byproduct of oxygen metabolism and a decrease in the ability of antioxidative compounds to detoxify cells and tissues. In addition to other factors (e.g., radiation, smoking, air pollution), dietary induced oxidative stress is one factor that can initiate and contribute to the immune cell activation and inflammation [88]. The immune cell activation, followed by a rise in oxygen consumption, also raises the amount of reactive oxygen species which can create an oxidative stress—inflammation cycle [88]. Thus, it is possible to say that the consequence of oxidative stress becomes its cause. The decreased antioxidative and detoxifying ability of the body can play a role in inflammation induced pain mechanisms [89]. Inversely, there is some evidence that an increased dietary antioxidant intake and the increased detoxifying ability of the body can alleviate pain among a chronic musculoskeletal pain population [90]. In the latest pain research, it is known that inflammation can interact with various pain mechanisms including nociceptive (pain arising due to activation of nociceptors), neuropathic (pain arising due to direct damage to the peripheral and central nervous system), and nociplastic (altered nociceptive system despite the absence of a clear nociceptive and neuropathic input) pain mechanisms [91].
Inflammation is the body’s immediate, natural, and protective response against infections and injuries. Physiologically, inflammatory processes, as a part of the immune reactions, are regulated by time. A late or prolonged inflammatory response might lose its protective effectiveness. A persistent proinflammatory state has been identified as an important risk factor for several pathophysiological conditions, including atherosclerosis, cardiovascular diseases, diabetes mellitus, obesity, cancer, and chronic pain [92]. Chronic and uncontrolled inflammation can be harmful and can lead to many acute and chronic diseases, including maintenance, occurrence, and prognosis of chronic pain [92]. This finding is supported by several pain studies on the immune system. To exemplify, immune cells evoke pain via the stimulation of nociceptors, changes in neuronal structures, and sensitization of the peripheral and central nervous systems via the release of inflammatory biomarkers [91,93].
Neuroinflammation is a localized inflammatory response that occurs in the peripheral and central nervous system [91]. In chronic pain conditions, neuroinflammation is characterized with the glial cell activation and an increased production of inflammatory biomarkers which can lead to peripheral and central nervous system sensitization [91]. Abnormal central nervous system glial cell activity has been reported in chronic pain conditions, especially nociplastic-related conditions, such as chronic non-specific low back pain, fibromyalgia, migraine, and spinal radiculopathy [94].
3.2. Microbiota-Gut-Brain Axis
Gut microbiota plays an important role in the human body and contributes to many structural, protective, and metabolic functions [95]. Thus, gut health constitutes an essential place in the maintenance of general health. The gut and brain have a bidirectional communication pathway and the intestinal microbiota has a modulating effect on this gut-brain axis [96]. The evidence shows that this link occurs through the connection between the vagus nerve and brainstem, via spinal afferents to the spinal cord [96]. Diversity of the gut microbiota is influenced by various factors, including medication use, mental health, infection, and nutrition which can lead to the dysregulation of the gut microbiota [97,98]. Dysregulation of the microbiota-gut-brain axis has been identified among various pathologic conditions, such as inflammatory bowel disease, diabetes, obesity, autism, depression, and chronic pain [97,98]. The accumulating evidence shows that the interrelation between nutrition and the microbiota-gut-brain axis can have a modulating effect in acute and chronic pain pathophysiology [97,98].
Microbes residing in the gut can be modified by nutritional factors. Thus, the microbiota-gut-brain axis has been identified as a target for nutritional interventions [99]. The differences in the diversity of the microbiome among the various populations that follow certain dietary patterns, such as vegetarian, vegan, and omnivorous diets, has been well documented [100]. Energy dense, unhealthy, proinflammatory dietary patterns that are nutrient poor and high in unsaturated fats, refined carbohydrates, and low in fruits and vegetables can cause a diet induced inflammation in the gut [94]. Proinflammatory cytokines released in response to unhealthy dietary patterns, activate the vagus nerve receptors located in the gastrointestinal tract. Upon activation, the vagus nerve can trigger the glial cell activation and the neuroinflammation process in the central nervous system [94]. Peripheral and central proinflammatory responses, including the aberrant glial cell activity, contribute to the maintenance, occurrence, and prognosis of chronic pain [94]. Targeting the gut microbiota with nutritional interventions in chronic pain populations is a promising approach for pain management.
3.3. Disturbed Glucose Metabolism
Diabetes has been reported as an important risk factor for chronic pain. In addition to neuropathic pain, chronic non-neuropathic pain conditions, such as fibromyalgia, chronic wide-spread pain, chronic low back, and neck pain are more common among people with diabetes, compared to people without diabetes, especially amongst those who have poorly controlled diabetes [101,102]. Patients with chronic musculoskeletal pain have been identified as having a disturbed glucose metabolism, including an increased fasting glucose level, an increased insulin resistance, a higher postprandial glycemic response, and a higher prevalence of type-2 diabetes mellitus [103]. A well-known antihyperglycemic medicine, metformin, which is commonly used to treat type-2 diabetes has also shown it can significantly alleviate pain in chronic pain populations and thus could be a potential treatment for people experiencing chronic pain [104].
An excessive carbohydrate intake and a decrease in glucose metabolism efficiency can increase reactive oxygen species and evoke an oxidative stress response [105]. The oxidative stress response is an endogenous factor that can activate toll like receptors and initiate inflammatory reactions in the peripheral and central nervous systems [105]. Thus, the identification of a disrupted glucose metabolism and targeting glucose regulation constitute significant places in chronic pain management [105]. Studies exploring the effect of low-carbohydrate diets, including the ketogenic diet, have revealed promising results including improvements in the glucose metabolism [106]. In another study, people with chronic musculoskeletal pain, who followed a low carbohydrate diet, had a decrease in serum inflammatory biomarkers and pain sensitivity [107]. Studies that explored the action mechanism of a ketogenic diet on chronic pain suggested that the carbohydrate intake played a role in neuroinflammation and central sensitization [108]. However, it is also important to consider the weight reducing effect of low-carbohydrate diets. A decrease in adipose tissue may also improve pain sensitivity in chronic pain populations and therefore, obesity requires special attention in terms of its role in the interaction between nutrition and pain generating mechanisms [6,109].
3.4. Disrupted Lipid Metabolism
Lipids are essential for several bodily functions, and are one of the body’s main energy sources. Nutrition strategies, including the modification of single nutrients, supplements, or overall eating patterns, can affect serum lipid profiles in both positive and negative ways. To exemplify, an excessive intake of saturated fats, dietary fructose, and an overall western style of eating increases low-density lipoprotein (LDL), triglycerides, and decreases high-density lipoprotein (HDL). A high LDL cholesterol level increases the risk of cardiovascular disease (CVD) and is commonly known as “bad cholesterol”, while a high HDL cholesterol level is protective, reduces the risk of CVD, and is often referred to as “good cholesterol”. Omega-3 unsaturated fatty acids, antioxidants, intermittent fasting, and adherence to the Mediterranean diet, can have the reverse effects on the same lipid biomarkers [110,111,112,113,114].
A disrupted lipid metabolism also plays a role in various health conditions, such as atherosclerosis, diabetes, cardiovascular diseases, metabolic syndrome, and obesity. [115]. The role of a disrupted lipid metabolism in chronic pain is gaining more attention and targeting this mechanism via dietary factors is a promising approach for chronic pain management. For instance, low back pain has been found to be prevalent among individuals with a decreased lumbar blood supply [116]. The relationship between the decreased lumbar blood supply and spinal pain constitutes a base for the atherosclerosis theory of the persistent non-specific low back pain. Prevalence of low back pain has been found inversely associated with the serum HDL cholesterol and positively associated with serum triglycerides and LDL cholesterol, which overall contribute to the atherosclerosis hypothesis [116,117]. Additionally, compared to healthy controls, fibromyalgia patients have shown a disrupted serum lipid profile and this disruption was found to be positively associated with pain sensitivity [118]. In a systematic review, the biomarkers of the serum lipid metabolism, including the decreased serum HDL cholesterol, the increased serum LDL cholesterol, and triglycerides, was found to be strongly associated with musculoskeletal pain arising from tendinopathy [119].
3.5. Obesity/Overweight
Obesity is associated with a proinflammatory state and is an important risk factor for various metabolic changes and chronic diseases, including cardiovascular diseases, cancer, diabetes mellitus, and chronic pain [109,120,121]. The existing evidence suggests that there is a concurrence and bidirectional relationship between obesity and chronic pain [109]. Obesity has been associated with several chronic musculoskeletal pain conditions including osteoarthritis, fibromyalgia, pelvic pain, and chronic low back pain [122]. It has been hypothesized that overweight/obesity play an important role in chronic pain by two main mechanisms; first increasing the mechanical load on neuromusculoskeletal structures and second, initiating or contributing to neuroimmune reactions, namely chronic low grade systemic inflammation [123].
Increased adipocytes and adipose tissue are positively associated with increased macrophages and promote inflammatory responses such as an increase in inflammatory cytokines (IL-6, TNF-alpha) and acute phase proteins (CRP) [124]. Excessive adipose tissue also increases the relocation of inflammatory cytokines into the central nervous system and promotes the activation of glial cells which can eventually play a role in nociplastic pain [125].
Exposure to high saturated fat and energy dense dietary patterns increase the circulated inflammatory cytokine levels. An in vivo study using an animal model suggests that exposure to a diet rich in saturated fat for one day causes the glial cell activation for two weeks in rats [126]. Dietary patterns that restrict the caloric intake have been shown to relieve pain in people with chronic musculoskeletal pain [127].
3.6. Epigenetic Factors
Epigenetics can be explained as a change in the gene expression without any change in the deoxyribonucleic acid (DNA) sequence. Epigenetic mechanisms are divided into three main categories, namely DNA methylation, histone modifications, and non-coding ribonucleic acid (RNA) interference [128]. Almost every cell in the body has the same DNA. However, each cell has different activated or highlighted genes in the DNA. Epigenetics explains the interaction between nature (genes) and nurture (environment), and how the genes we inherit interact with environmental factors including diet [128]. Many epigenetic mechanisms are reversible and modifiable which make them an attractive therapeutic target.
Nutrition is a major modifiable lifestyle factor that has the ability to alter the epigenetic regulation and can cause an epigenetic dysregulation. Additionally, epigenetic markers also have the ability to alter the body’s response to certain dietary intake and patterns [129,130]. Dysregulation of the epigenetic markers can alter the gene expression, protein synthesis, cell function, and metabolism and can lead to chronic diseases [131,132].
Recent findings show that epigenetic changes can alter the expression of nociceptive or antinociceptive genes [133]. Moreover, the epigenetic dysregulation can play a role in the transition from acute to chronic pain [134]. Preclinical studies have shown an increase in inflammatory responses after the consumption of a diet rich in saturated fats and a high-carbohydrate diet via DNA methylation [135]. The DNA methylation level of genes that promote inflammatory cytokines, especially TNF-alpha, has been associated with obesity and an omega-6 polyunsaturated fatty acids intake [136]. Saturated fatty acids are known for their inflammatory characteristics and a higher intake of saturated fatty acids has been associated with the DNA methylation level of the genes that play an essential role in the inflammatory biomarker synthesis and insulin resistance [137]. Alternatively, nutrients and foods with anti-inflammatory properties, such as omega-3 polyunsaturated fatty acids, extra virgin olive oil, curcumin, and polyphenols showed anti-inflammatory effects via its effects on the DNA methylation processes in immune cells [138]. Early findings show that there is an interaction between nutrition and the epigenetic factors and this has an important role in chronic pain and the associated mechanisms, such as obesity, a disturbed glucose metabolism, and gut microbiota diversity. Although the use of genetic and epigenetic data in chronic pain management is still in a very early phase, the potential for the development of personalized pain medicine, or precision pain medicine is both promising and innovative [139].
4. Implementation and Scope of Nutrition in Chronic Pain Management
4.1. Nutrition Assessment for Chronic Pain
There is growing evidence to show that there is an association between diet and health outcomes that are important for people experiencing chronic pain. Therefore, a nutrition assessment should be conducted early in treatment. This may be through a brief, opportunistic intervention that a health professional (e.g., general practitioner (GP), nurse or allied health professional) may provide to a patient, a structured nutrition screening process at a pain clinic, or a comprehensive dietary assessment conducted by a dietitian. There are several nutrition-related risk factors associated with chronic pain and these should be addressed in a dietary assessment. The factors include malnutrition, weight change, the presence of other comorbidities, abnormal biochemistry results, appetite or gastrointestinal complaints, and a poor dietary intake.
4.2. Nutrition Treatments for Chronic Pain
Evidence suggests that following a predominately plant-based eating pattern (e.g., vegetarian, vegan, or flexitarian eating pattern) or a Mediterranean eating pattern (characterized by a high consumption of fruit, vegetables, legumes, wholegrains, dairy, olive oil, moderate consumption of fish, and small amounts of red meat) or an optimizing diet quality are most effective at reducing pain experiences [53]. The evidence available in the scientific literature is also supported by practice guidance toolkits [18]. However, these guidelines are limited to specific chronic pain conditions such as osteoarthritis, rheumatoid arthritis, and fibromyalgia. These toolkits also recommend predominately plant-based eating patterns, healthy fats and oils, and consuming a wide variety of nutritious foods. The evidence presented in the literature and toolkits can be synthesized into dietary recommendations that health professionals can provide to people experiencing pain.
It is also important to consider the barriers or practical implications to adhering to a particular eating pattern. These include: ability and access to shop, prepare and cook food, pain flare-ups, cost, culinary skills, sleep, gastrointestinal symptoms, food intolerances, environment, motivation, and mood [53,175]. As part of a multidisciplinary team, a dietitian can work with the patient and their health care team to develop a sustainable plan that improves pain experiences, other health outcomes, and that can be adhered to over a long period of time [175].
Social determinants of health, such as education, socioeconomic status, access and quality of essential services, and the social environment, also play a role in an individual’s ability to access nutritious and affordable food. Food insecurity is the inability to reliably access adequate and affordable nutritious food and it is associated with chronic pain and poor mental health. Findings from a recent survey of 200 adult food bank users in the United States, found that 53% of respondents reported experiencing chronic pain [176]. In this study, after controlling for age and gender, depression, and chronic pain significantly predicted food insecurity. A study which analyzed data from approximately 80,000 Canadians aged ≥12 years found that those who were food-insecure were 1.3 times more likely to experience chronic pain and almost 2.7 times more likely to have used prescription opioids in the last year [177]. This demonstrates that multidisciplinary teams must explore barriers, practical implications, and social determinants of health when it comes to nutrition and pain.
Other health professionals, such as psychologists, occupational therapists, and physiotherapists can also provide valuable advice and guidance that will work, in combination with the advice and guidance provided by the dietitian, to address some of these practical implications. For example, a psychologist can help address mood and motivation, an occupational therapist can undertake a functional assessment and provide advice on how to participate in nutrition and food-related activities, such as cooking, and physiotherapists can assist by facilitating people to build their strength and mobility which will help with accessing food.
A common denominator for all health professionals is behavior change. These practical implications can also be considered barriers that may make behavior change difficult. Behavior change is a fundamental part of the biopsychosocial and lifestyle approaches to pain management. Models and frameworks, such as the Behavior Change Model [178], can be used to understand and implement behavior change to overcome these barriers. It is important that all health professionals in a multidisciplinary team are familiar with behavior change models and incorporate behavior change techniques in their practice.
4.3. Scope of Practice
Dietary changes vary in their simplicity and sustainability. Some changes are easy, and others are harder to implement and sustain over time. These changes can be categorized into general healthy eating, basic, or complex recommendations for chronic pain, and personalized medical nutrition therapy as outlined in Figure 3.
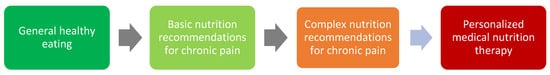
Figure 3. Nutrition and the chronic pain scope of practice.
In a multidisciplinary team, all health professionals should understand all of the components involved in pain management, including nutrition. However, it must be acknowledged that all health professionals have a particular area of expertise. Dietitians are experts qualified to provide medical nutrition therapy using the nutrition care process. In the nutrition and chronic pain scope of practice, all health professionals, should understand general healthy eating and have a basic understanding of nutrition-related recommendations for chronic pain. Pain management teams include medical, nursing, physiotherapy, psychology, and other allied health professionals and all have a significant role in providing relevant and appropriate health education to patients, including nutritional recommendations. However, a comprehensive understanding of nutrition-related recommendations for chronic pain and personalized medical nutrition therapy, should be provided by credentialed dietitians (e.g., Accredited Practising Dietitian or Registered Dietitian) who have undertaken approved study at university and registered with their respective national dietetic association (e.g., Dietitian’s Australia or British Dietetic Association).
This entry is adapted from the peer-reviewed paper 10.3390/jcm11195950
This entry is offline, you can click here to edit this entry!
