Nanoscience and nanotechnology explore the properties and application of particulate systems at the nanometric size. At this scale, materials exhibit properties and characteristics different from their bulk form due to the surface and quantum confinement effects. These effects are related to the increase in the area/volume ratio, which can improve textural properties, such as specific surface area and porosity. Additionally, quantum effects are involved with electronic and optical modifications. When considering their unique properties and characteristics, nanoparticles and nanomaterials have been widely used in water remediation, pesticide detection, and especially in biomedical applications. Despite their excellent properties, the toxicity of nanoparticles (NPs) is a common concern for the scientific community.
- magnetic nanoparticles
- multifunctional systems
- nanocarriers
1. Biomolecule Purification
2. Food Packaging
3. Antimicrobial Activity
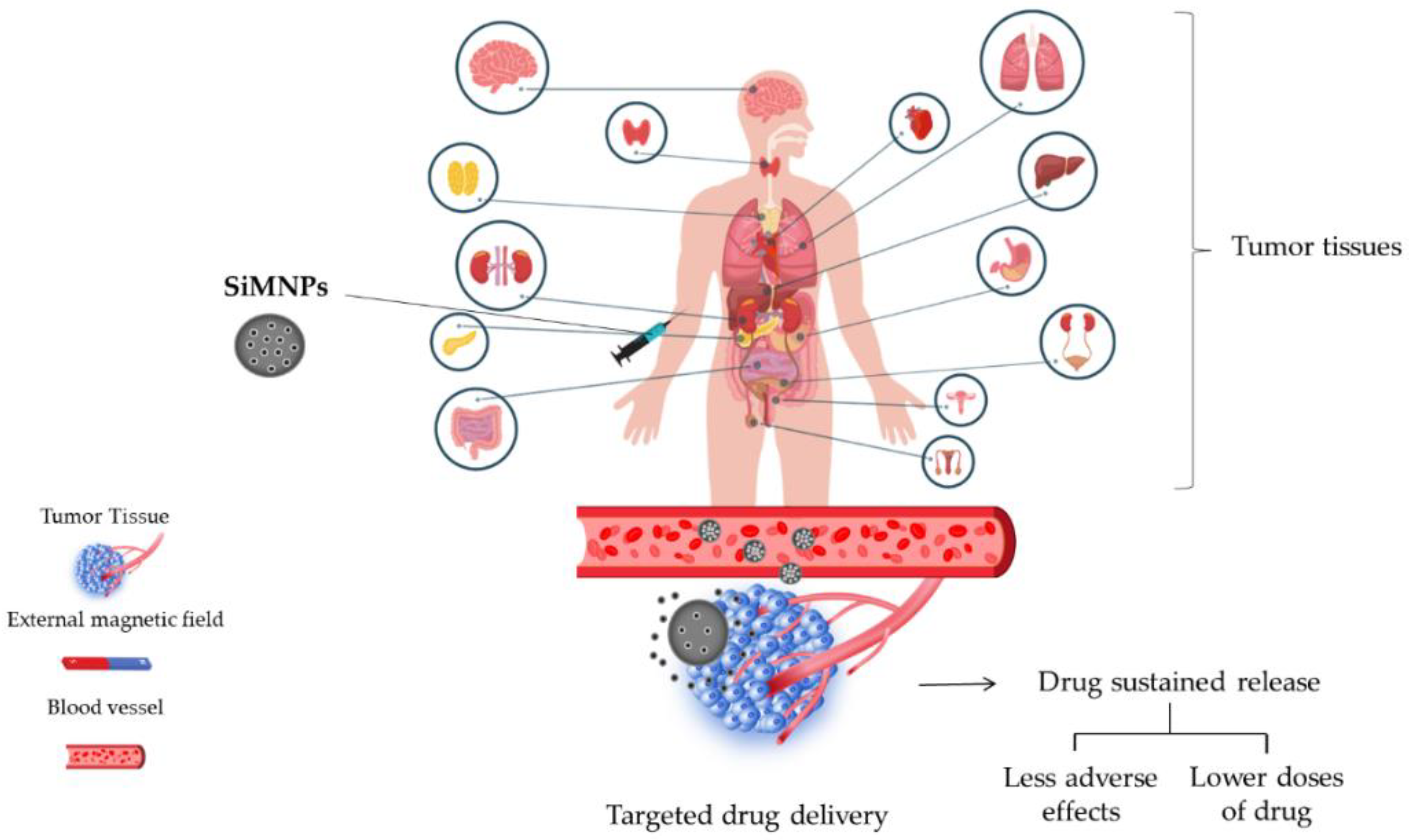
4. Drug Delivery and Release Studies
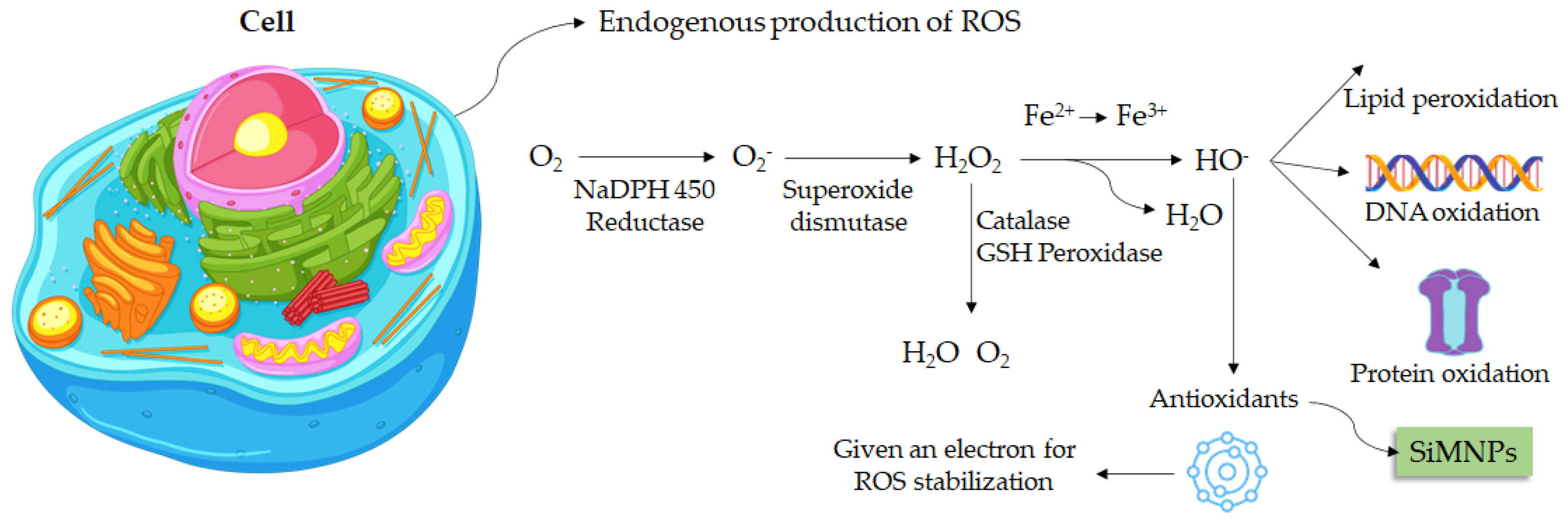
5. Antioxidant Activity
6. Silica-Based Nanoparticles Biocompatibility and Safe Profile Assessment
7. Silica Nanoparticles Carriers Focusing on Cardiovascular Treatment
8. Silica Nanoparticles against Cancer Cells

9. Point-of-Care Detection of Silica-Based Nanoparticles
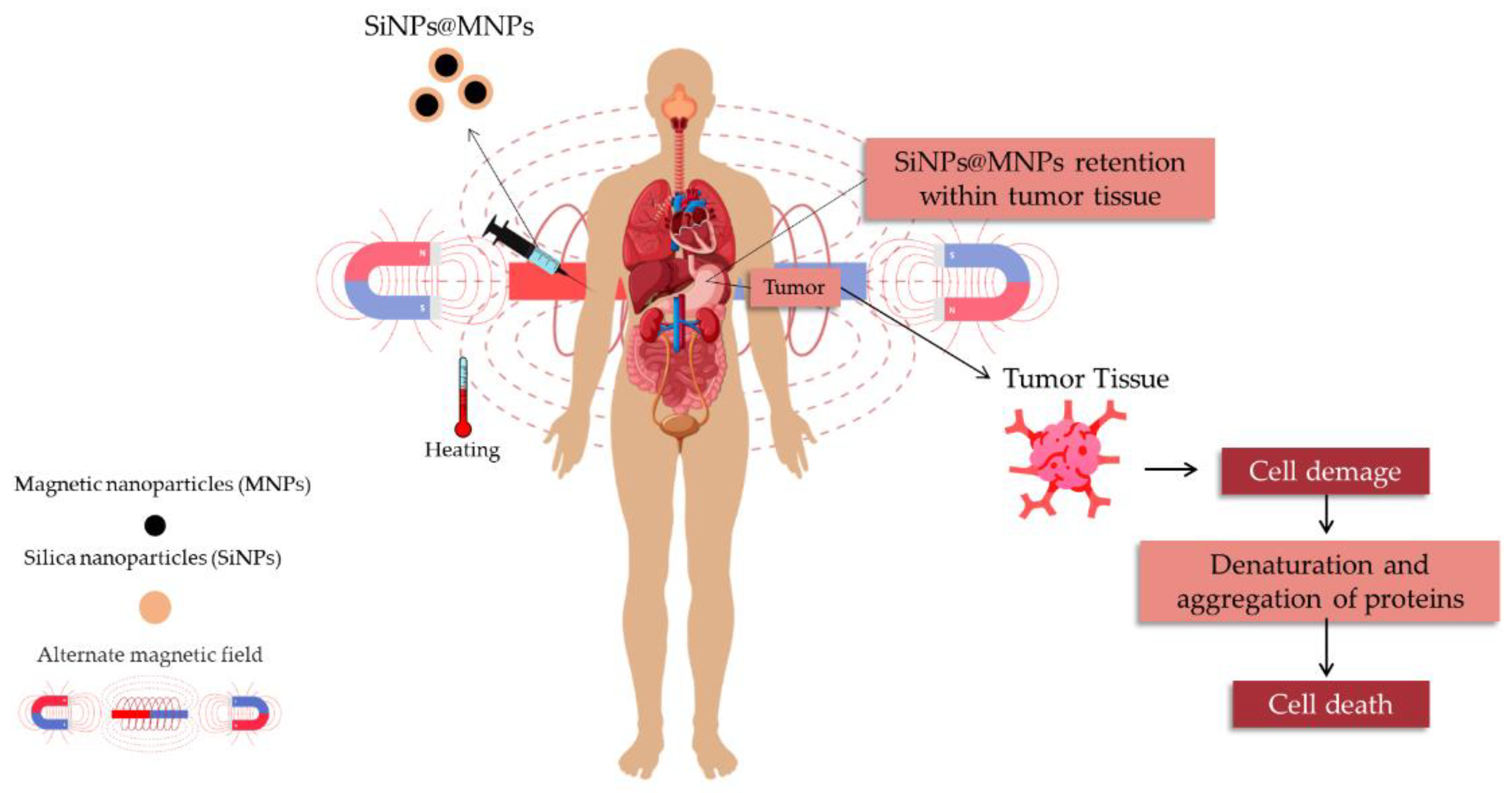
Magnetic Hyperthermia Therapy and Magnetic Resonance Imaging
This entry is adapted from the peer-reviewed paper 10.3390/magnetochemistry8100131
References
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394.
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, haracterization, and purification. Phytochemistry 2018, 154, 94–105.
- Rhoden, C.R.B.; da Silva Bruckmann, F.; da Rosa Salles, T.; Junior, C.G.K.; Mortari, S.R. Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J. Water Process Eng. 2021, 43, 102262.
- Sánta-Bell, E.; Molnár, Z.; Varga, A.; Nay, F.; Hornyánsky, G.; Paizs, C.; Balogh, D.; Poppe, L. “Fishing and Hunting”—Selective Immobilization of a Recombinant Phenylalanine Ammonia-Lyase from Fermentation Media. Molecules 2019, 24, 4146.
- Niu, C.; Song, X.; Zhang, Y.; Dai, L.; Wei, J.; Yue, T.; Song, Z. A rapid one-step process for the isolation of antibacterial peptides by silica-decorated Fe3O4 nanoparticles. LWT 2022, 155, 112858.
- Benelmekki, M.; Xuriguera, E.; Caparros, C.; Rodríguez-Carmona, E.; Mendoza, R.; Corchero, J.L.; Lanceros-Mendez, S.; Martinez, L.M. Design and characterization of Ni2+ and Co2+ decorated Porous Magnetic Silica spheres synthesized by hydrothermal-assisted modified-Stöber method for His-tagged proteins separation. J. Colloid Interf. Sci. 2012, 365, 156–162.
- Liu, S.; Chen, H.; Lu, X.; Deng, C.; Zhang, X.; Yang, P. Facile synthesis of copper (II) immobilized on magnetic mesoporous silica microspheres for selective enrichment of peptides for mass spectrometry analysis. Angew. Chem. Int. Ed. 2010, 49, 7557–7561.
- Surendhiran, D.; Roy, V.C.; Park, J.S.; Chun, B.S. Fabrication of chitosan-based food packaging film impregnated with turmeric essential oil (TEO)-loaded magnetic-silica nanocomposites for surimi preservation. Int. J. Biol. Macromol. 2022, 203, 650–660.
- Da Silva Bruckmann, F.; Viana, A.R.; Lopes, L.Q.S.; Santos, R.C.V.; Muller, E.I.; Mortari, S.R.; Rhoden, C.R.B. Synthesis, characterization, and biological activity evaluation of magnetite-functionalized eugenol. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1459–1472.
- Deon, M.; Morawski, F.M.; Passaia, C.; Dalmás, M.; Laranja, D.C.; Malheiros, P.S.; Arenas, L.T.; Costa, T.M.H.; de Menezes, E.W.; Benvenutti, E.V. Chitosan-stabilized gold nanoparticles supported on silica/titania magnetic xerogel applied as antibacterial system. J. Sol-Gel Sci. Technol. 2019, 89, 333–342.
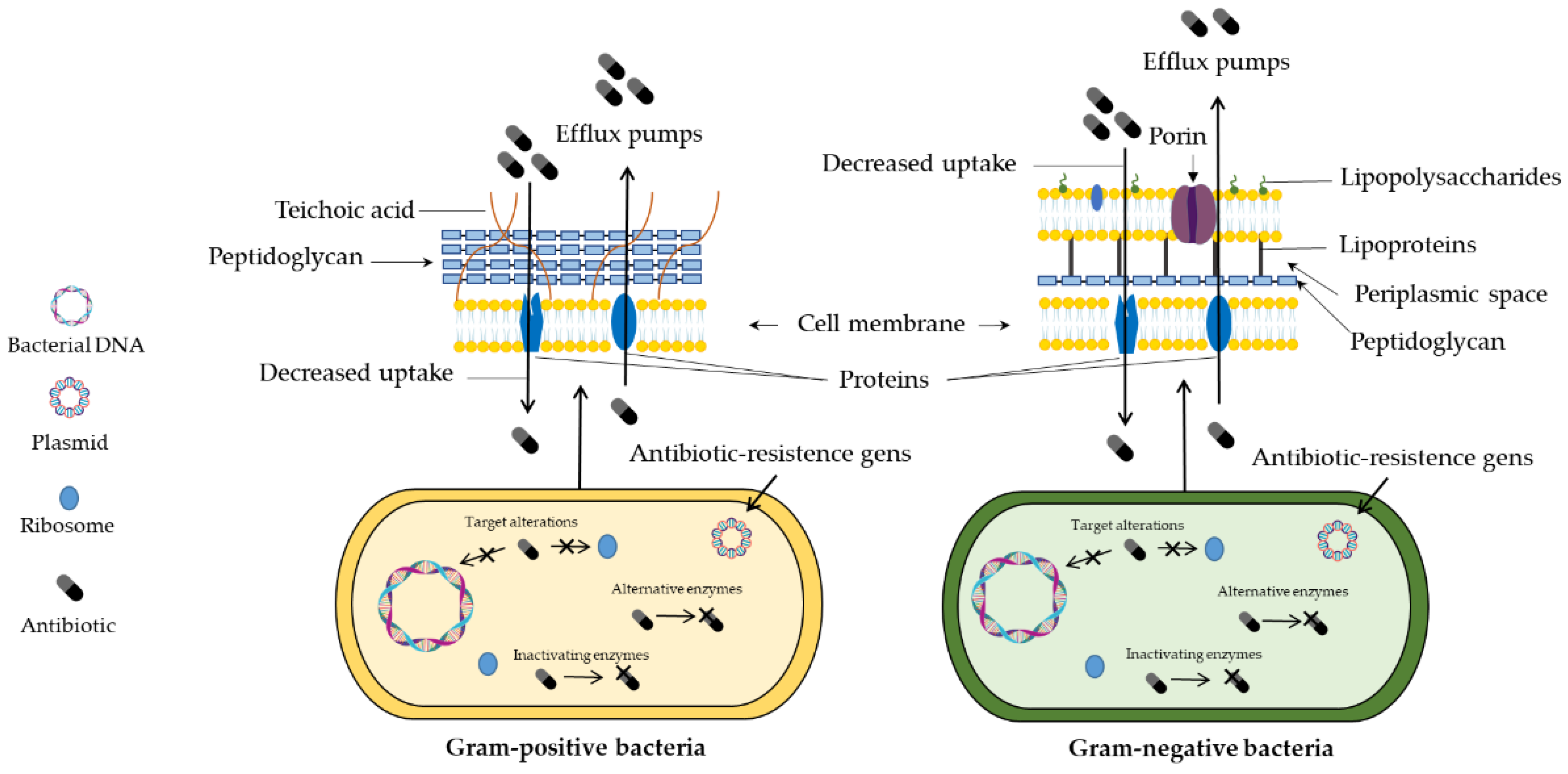
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482.
- Da Rosa Salles, T.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713.
- Follmann, H.D.; Oliveira, O.N.; Lazarin-Bidóia, D.; Nakamura, C.V.; Huang, X.; Asefa, T.; Silva, R. Multifunctional hybrid aerogels: Hyperbranched polymer-trapped mesoporous silica nanoparticles for sustained and prolonged drug release. Nanoscale 2018, 10, 1704–1715.
- Nayeem, J.; Al-Bari, M.A.A.; Mahiuddin, M.; Rahman, M.A.; Mefford, O.T.; Ahmad, H.; Rahman, M.M. Silica coating of iron oxide magnetic nanoparticles by reverse microemulsion method and their functionalization with cationic polymer P (NIPAm-co-AMPTMA) for antibacterial vancomycin immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125857.
- Zhang, W.; Taheri-Ledari, R.; Hajizadeh, Z.; Zolfaghari, E.; Ahghari, M.R.; Maleki, A.; Hamblin, M.R.; Tian, Y. Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale 2020, 12, 3855–3870.
- Shahabadi, N.; Akbari, A.; Karampour, F.; Falsafi, M. Cytotoxicity and antibacterial activities of new chemically synthesized magnetic nanoparticles containing eugenol. J. Drug Deliv. Sci. Technol. 2019, 49, 113–122.
- Chen, Y.; Feng, C.; Zhang, Q.; Luo, M.; Xu, J.; Han, Q. Engineering of antibacterial/recyclable difunctional nanoparticles via synergism of quaternary ammonia salt site and N-halamine sites on magnetic surface. Colloids Surf. B Biointerfaces 2020, 187, 110642.
- Nazari, S.; Gholami, M.; Farzadkia, M.; Dourbash, F.A.; Arzanlou, M.; Kalantary, R.R. Synthesis and evaluation of the antibacterial effect of silica-coated modified magnetic poly-(amidoamine) G5 nanoparticles on E. coli and S. aureus. J. Mol. Liq. 2019, 276, 93–104.
- Shatan, A.B.; Venclíková, K.; Zasońska, B.A.; Patsula, V.; Pop-Georgievski, O.; Petrovský, E.; Horák, D. Antibacterial silver-conjugated magnetic nanoparticles: Design, synthesis and bactericidal effect. Pharm. Res. 2019, 36, 147.
- Wang, X.; Sun, W.; Yang, W.; Gao, S.; Sun, C.; Li, Q. Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 2019, 1, 840–848.
- Ahmadi, A.; Sedaghat, T.; Motamedi, H.; Azadi, R. Anchoring of Cu (II)-Schiff base complex on magnetic mesoporous silica nanoparticles: Catalytic efficacy in one-pot synthesis of 5-substituted-1H-tetrazoles, antibacterial activity evaluation and immobilization of α-amylase. Appl. Organomet. Chem. 2020, 34, e5572.
- Han, Q.; Wang, X.; Liu, X.; Xiao, W.; Cai, S.; Wang, C.; Yang, R. Controllable fabrication of magnetic core–shell nanocomposites with high peroxide mimetic properties for bacterial detection and antibacterial applications. J. Mater. Chem. B 2019, 7, 1124–1132.
- Chanhom, P.; Charoenlap, N.; Tomapatanaget, B.; Insin, N. Colloidal titania-silica-iron oxide nanocomposites and the effect from silica thickness on the photocatalytic and bactericidal activities. J. Magn. Magn. Mater. 2017, 427, 54–59.
- El Nahrawy, A.M.; Mansour, A.M.; Elzwawy, A.; Abou Hammad, A.B.; Hemdan, B.A. Spectroscopic and magnetic properties of Co0.15Al0.25−xNi0.6+xFe2O4 nanocomposites aided by silica for prohibiting pathogenic bacteria during sewage handling. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100672.
- Balasamy, R.J.; Ravinayagam, V.; Alomari, M.; Ansari, M.A.; Almofty, S.A.; Rehman, S.; Dafalla, H.; Marimuthu, P.R.; Akhtar, S.; Al Hamad, M. Cisplatin delivery, anticancer and antibacterial properties of Fe/SBA-16/ZIF-8 nanocomposite. RSC Adv. 2019, 9, 42395–42408.
- González-Sarrías, A.; Tomé-Carneiro, J.; Bellesia, A.; Tomás-Barberán, F.A.; Espín, J.C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 2015, 6, 1460–1469.
- Jiang, A.; Song, B.; Ji, X.; Peng, F.; Wang, H.; Su, Y.; He, Y. Doxorubicin-loaded silicon nanoparticles impregnated into red blood cells featuring bright fluorescence, strong photostability, and lengthened blood residency. Nano Res. 2018, 11, 2285–2294.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51.
- Pinna, A.; Migheli, R.; Rocchitta, G.; Serra, P.A.; Falcaro, P.; Malfatti, L.; Innocenzi, P. A MOF-based carrier for in situ dopamine delivery. RSC Adv. 2018, 8, 25664–25672.
- Pon-On, W.; Tithito, T.; Maneeprakorn, W.; Phenrat, T.; Tang, I.M. Investigation of magnetic silica with thermoresponsive chitosan coating for drug controlled release and magnetic hyperthermia application. Macromol. Mater. Eng. 2019, 97, 23–30.
- Tran, V.A.; Shim, K.; Lee, S.W.; An, S.S.A. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int. J. Nanomed. 2020, 15, 7667.
- Liu, J.; Liu, W.; Zhang, K.; Shi, J.; Zhang, Z. A magnetic drug delivery system with “OFF–ON” state via specific molecular recognition and conformational changes for precise tumor therapy. Adv. Healthc. Mater. 2020, 9, 1901316.
- Espinoza, M.J.; Lin, K.-S.; Weng, M.-T.; Kunene, S.C.; Wang, S.-S.S. In Vitro Studies of Pluronic F127 Coated Magnetic Silica Nanocarriers for Drug Delivery System Targeting Liver Cancer. Eur. Polym. J. 2021, 153, 110504.
- Wu, J.; Jiang, W.; Shen, Y.; Jiang, W.; Tian, R. Synthesis and Characterization of Mesoporous Magnetic Nanocomposites Wrapped with Chitosan Gatekeepers for PH-Sensitive Controlled Release of Doxorubicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 132–140.
- Moorthy, M.S.; Subramanian, B.; Panchanathan, M.; Mondal, S.; Kim, H.; Lee, K.D.; Oh, J. Fucoidan-coated core–shell magnetic mesoporous silica nanoparticles for chemotherapy and magnetic hyperthermia-based thermal therapy applications. New J. Chem. 2017, 41, 15334–15346.
- Fan, H.; Xing, X.; Yang, Y.; Li, B.; Wang, C.; Qiu, D. Triple function nanocomposites of porous silica-CoFe2O4-MWCNTs as a carrier for pH-sensitive anti-cancer drug controlled delivery. Dalton Trans. 2017, 46, 14831–14838.
- Wang, X.; Yin, H.; Guan, Y.; Yang, Y.; Huang, Y.; Yuan, H.; Ni, C. Graphene Oxide Covalently Grafted Nanoparticles for Epirubicin Loading and Releasing. J. Nanosci. Nanotechnol. 2020, 20, 2104–2113.
- Wang, C.; Zhao, N.; Huang, Y.; He, R.; Xu, S.; Yuan, W. Coordination of injectable self-healing hydrogel with Mn-Zn mesoporous silica nanospheres for tumor MR imaging and efficient synergistic magnetothermal-chemo-chemodynamic therapy. Chem. Eng. Technol. 2020, 401, 126100.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 2, 689–709.
- Halevas, E.; Mavroidi, B.; Nday, C.M.; Tang, J.; Smith, G.C.; Boukos, N.; Litsardakis, G.; Pelecanou, M.; Salifoglou, A. Modified magnetic core-shell mesoporous silica nano-formulations with encapsulated quercetin exhibit anti-amyloid and antioxidant activity. J. Inorg. Biochem. 2020, 213, 111271.
- Świętek, M.; Ma, Y.H.; Wu, N.P.; Paruzel, A.; Tokarz, W.; Horák, D. Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects. Nanomaterials 2022, 12, 1310.
- Zare, M.; Sarkati, M.N. Chitosan-functionalized Fe3O4 nanoparticles as an excellent biocompatible nanocarrier for silymarin delivery. Polym. Adv. Technol. 2021, 32, 4094–4100.
- Patsula, V.; Moskvin, M.; Siow, W.X.; Konefal, R.; Ma, Y.H.; Horák, D. Antioxidant polymer-modified maghemite nanoparticles. J. Magn. Magn. Mater. 2019, 473, 517–526.
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. Magnetite mesoporous silica nanoparticles embedded in carboxybetaine methacrylate for application in hyperthermia and drug delivery. New J. Chem. 2020, 44, 8232–8240.
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; Di Gioia, C.; Carletti, R.; Lisi, A. Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine. Nanoscale 2020, 12, 1759–1778.
- Janßen, H.C.; Angrisani, N.; Kalies, S.; Hansmann, F.; Kietzmann, M.; Warwas, D.P.; Behrens, P.; Reifenrath, J. Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics. J. Nanobiotechnol. 2020, 18, 14.
- Nasiri, R.; Dabagh, S.; Meamar, R.; Idris, A.; Muhammad, I.; Irfan, M.; Nodeh, H.R. Papain grafted into the silica coated iron-based magnetic nanoparticles ‘ SiO2-PPN’as a new delivery vehicle to the HeLa cells. Nanotechnology 2020, 31, 195603.
- Rascol, E.; Pisani, C.; Dorandeu, C.; Nyalosaso, J.L.; Charnay, C.; Daurat, M.; Da Silva, A.; Devoisselle, J.-M.; Gaillard, J.-C.; Armengaud, J.; et al. Biosafety of mesoporous silica nanoparticles. Biomimetics 2018, 3, 22.
- Navarro-Palomares, E.; González-Saiz, P.; Renero-Lecuna, C.; Martín-Rodríguez, R.; Aguado, F.; González-Alonso, D.; Valiente, R. Dye-doped biodegradable nanoparticle SiO 2 coating on zinc-and iron-oxide nanoparticles to improve biocompatibility and for in vivo imaging studies. Nanoscale 2020, 12, 6164–6175.
- Sheng, Y.; Li, S.; Duan, Z.; Zhang, R.; Xue, J. Fluorescent magnetic nanoparticles as minimally-invasive multi-functional theranostic platform for fluorescence imaging, MRI and magnetic hyperthermia. Mater. Chem. Phys. 2018, 204, 388–396.
- Huang, J.; Sun, C.; Yao, D.; Wang, C.Z.; Zhang, L.; Zhang, Y.; Yuan, C.S. Novel surface imprinted magnetic mesoporous silica as artificial antibodies for efficient discovery and capture of candidate nNOS–PSD-95 uncouplers for stroke treatment. J. Mater. Chem. 2018, 6, 1531–1542.
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/Shell Nanoparticles in Biomedical Applications. Adv. Colloid Interface Sci. 2014, 209, 8–39.
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Lee, K.D.; Oh, J. Crown ether triad modified core–shell magnetic mesoporous silica nanocarrier for pH-responsive drug delivery and magnetic hyperthermia applications. New J. Chem. 2017, 41, 10935–10947.
- Itatahine, A.; Mehdi, Y.A.; Fizir, M.; Qi, M.; Dramou, P.; He, H. Multifunctional carbon nanomateriels for camptothecine low-water soluble anticancer drug delivery. New J. Chem. 2018, 42, 1326–1336.
- Gao, Q.; Xie, W.; Wang, Y.; Wang, D.; Guo, Z.; Gao, F.; Cai, Q. A theranostic nanocomposite system based on radial mesoporous silica hybridized with Fe 3 O 4 nanoparticles for targeted magnetic field responsive chemotherapy of breast cancer. RSC Adv. 2018, 8, 4321–4328.
- da Silva Bruckmann, F.; Pimentel, A.C.; Viana, A.R.; da Rosa Salles, T.; Krause, L.M.F.; Mortari, S.R.; Silva, I.Z.; Rhoden, C.R.B. Synthesis, characterization and cytotoxicity evaluation of magnetic nanosilica in L929 cell line. Discip. Sci. Sér. Ciên. Nat. Tecnol. 2020, 21, 1–14.
- Abbasi Kajani, A.; Bordbar, A.-K.; Zarkesh-Esfahani, S.H.; Razmjou, A.; Hou, J. Gold/Silver Decorated Magnetic Nanostructures as Theranostic Agents: Synthesis, Characterization and in-Vitro Study. J. Mol. Liq. 2017, 247, 238–245.
- Taher, Z.; Legge, C.; Winder, N.; Lysyganicz, P.; Rawlings, A.; Bryant, H.; Staniland, S. Magnetosomes and magnetosome mimics: Preparation, cancer cell uptake and functionalization for future cancer therapies. Pharmaceutics 2021, 13, 367.
- Akhtar, S.; Gunday, S.T.; Alqosaibi, A.I.; Aldossary, H.; Bozkurt, A.; Khan, F.A. Template-free preparation of iron oxide loaded hollow silica spheres and their anticancer proliferation capabilities. RSC Adv. 2022, 12, 6791–6802.
- Foglia, S.; Ledda, M.; Fioretti, D.; Iucci, G.; Papi, M.; Capellini, G.; Lisi, A. In vitro biocompatibility study of sub-5 nm silica-coated magnetic iron oxide fluorescent nanoparticles for potential biomedical application. Sci. Rep. 2017, 7, 1–13.
- Solak, K.; Mavi, A.; Yılmaz, B. Disulfiram-loaded functionalized magnetic nanoparticles combined with copper and sodium nitroprusside in breast cancer cells. Mater. Sc. Eng. C 2021, 119, 111452.
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Shadjou, N.; Jouyban, A. Probing the Specific Binding of Folic Acid to Folate Receptor Using Amino-Functionalized Mesoporous Silica Nanoparticles for Differentiation of MCF 7 Tumoral Cells from MCF 10A. Biosens. Bioelectron. 2018, 115, 61–69.
- Monaco, I.; Armanetti, P.; Locatelli, E.; Flori, A.; Maturi, M.; Del Turco, S.; Franchini, M.C. Smart assembly of Mn-ferrites/silica core–shell with fluorescein and gold nanorods: Robust and stable nanomicelles for in vivo triple modality imaging. J. Mater. Chem. 2018, 6, 2993–2999.
- Cabrera-García, A.; Checa-Chavarria, E.; Pacheco-Torres, J.; Bernabeu-Sanz, Á.; Vidal-Moya, A.; Rivero-Buceta, E.; Sastre, G.; Fernández, E.; Botella, P. Engineered contrast agents in a single structure for T1–T2 dual magnetic resonance imaging. Nanoscale 2018, 10, 6349–6360.
- Zhuang, H.; Xu, C.; Gao, F.; Li, Y.; Lei, C.; Yu, C. Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay. Bioengineering 2022, 9, 266.
- Liao, S.H.; Liu, C.H.; Bastakoti, B.P.; Suzuki, N.; Chang, Y.; Yamauchi, Y.; Lin, F.L.; Wu, K.C. Functionalized magnetic iron oxide/alginate core-shell nanoparticles for targeting hyperthermia. Int. J. Nanomed. 2015, 10, 3315.
- Nunes, F.B.; Da Silva Bruckmann, F.; Da Rosa Salles, T.; Rhoden, C.B.R. Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Environ. Sci. Pollut. Res. 2022, 1–14.
- Da Silva Bruckmann, F.; Ledur, C.M.; Da Silva, I.Z.; Dotto, G.L.; Rhoden, C.R.B. A DFT theoretical and experimental study about tetracycline adsorption onto magnetic graphene oxide. J Mol. Liq. 2022, 353, 118837.
- Bruckmann, F.D.S.; Rossato Viana, A.; Tonel, M.Z.; Fagan, S.B.; Garcia, W.J.D.S.; Oliveira, A.H.D.; Rhoden, C.R. B Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Environ. Sci. Pollut. Res. 2022, 29, 70413–70434.
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.; Ivkov, R. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779.
- Rodríguez-Galván, A.; Rivera, M.; García-López, P.; Medina, L.A.; Basiuk, V.A. Gadolinium-containing carbon nanomaterials for magnetic resonance imaging: Trends and challenges. J. Cell. Mol. Med. 2020, 24, 3779–3794.
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398.
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic nanoparticles as MRI contrast agents. Top. Curr. Chem. 2020, 378, 49–91.
- Weissleder, R.; Cheng, H.C.; Bogdanova, A.; Bogdanov, A., Jr. Magnetically labeled cells can be detected by MR imaging. J. Magn. Reson. Imaging 1997, 7, 258–263.
- Benyettou, F.; Das, G.; Nair, A.R.; Prakasam, T.; Shinde, D.B.; Sharma, S.K.; Whelan, J.; Lalatonne, Y.; Traboulsi, H.; Pasricha, R.; et al. Covalent organic framework embedded with magnetic nanoparticles for MRI and chemo-thermotherapy. J. Am. Chem. Soc. 2020, 142, 18782–18794.
- Rümenapp, C.; Gleich, B.; Haase, A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm. Res. 2012, 29, 1165–1179.
- Kubíčková, L.; Kaman, O.; Veverka, P.; Herynek, V.; Brázda, P.; Vosmanská, M.; Kohout, J. The ε-AlxFe2-xO3 nanomagnets as MRI contrast agents: Factors influencing transverse relaxivity. Colloids Surf. 2020, 589, 124423.
- Kavkhani, R.; Hajalilou, A.; Abouzari-Lotf, E.; Ferreira, L.P.; Cruz, M.M.; Yusefi, M.; Ismail, U.N. CTAB assisted synthesis of MnFe2O4@ SiO2 nanoparticles for magnetic hyperthermia and MRI application. Mater. Today Commun. 2022, 31, 103412.
- Iliasov, A.R.; Nizamov, T.R.; Naumenko, V.A.; Garanina, A.S.; Vodopyanov, S.S.; Nikitin, A.A.; Abakumov, M.A. Non-magnetic shell coating of magnetic nanoparticles as key factor of toxicity for cancer cells in a low frequency alternating magnetic field. Colloids. Surf. B 2021, 206, 111931.
- Yang, T.; Niu, D.; Chen, J.; He, J.; Yang, S.; Jia, X.; Li, Y. Biodegradable organosilica magnetic micelles for magnetically targeted MRI and GSH-triggered tumor chemotherapy. Biomater. Sci. 2019, 7, 2951–2960.
