Bacteriophage-based biosensors offer several benefits, including specificity to their host organism, the detection of only live pathogens, and resistance to extreme environmental factors such as organic solvents, high temperatures, and a wide pH range. Phage-based biosensors are receiving increasing attention owing to their high degree of accuracy, specificity, and reduced assay times. These characteristics, coupled with their abundant supply, make phages a novel bio-recognition molecule in assay development, including biosensors for the detection of foodborne bacterial pathogens to ensure food safety.
1. Introduction
Foodborne microorganisms are an important cause of human illnesses worldwide. Two-thirds of human foodborne diseases are caused by bacterial pathogens throughout the globe, especially in developing nations [
1]. The most commonly encountered foodborne bacterial pathogens are
Staphylococcus aureus (
S. aureus),
Salmonella enterica serovar Typhimurium (
S. Typhimurium),
Clostridium perfringens (
C. perfringens),
Campylobacter species,
Escherichia coli (
E. coli), and
Listeria monocytogenes (
L. monocytogenes). Most of these organisms have zoonotic importance, causing huge adverse effects to both public health and economic sectors [
1]. Of these bacterial foodborne pathogens, human-sourced pathogens such as
E. coli and
Salmonella Typhi can contaminate the food supply chain through the feces of infected individuals [
2], while many others such as non-typhoidal
Salmonella,
Campylobacter,
Staphylococcus,
Yersinia,
Clostridium, and
Listeria are transmitted through food animals, poultry, milk, or eggs [
3]. Environmental transmission has been frequently reported for several of the pathogens, including
Salmonella,
E. coli O157:H7, and
Campylobacter, during pre- and post-harvest food processing, storage, and transportation [
4]. The Centers for Disease Control and Prevention (CDC) routinely monitors the presence of these pathogens in food [
5]. The US FDA (Food and Drug Administration) and FSIS (Food Safety Inspection Service) agencies strictly regulate their presence in raw or ready-to-eat products [
5]; therefore, reliable detection methods that are capable of detecting live pathogens are critical.
Conventional foodborne pathogen detection methods mainly depend on specific biochemical, serological, and nucleic-acid-based techniques [
6,
7]. These methods require skilled technicians and are time-consuming, expensive, and difficult to interpret. Most rapid detection methods cannot distinguish dead from live cells unless a growth-based enrichment step is used, making them inapplicable in many food processing facilities [
8]. Conversely, enzyme-linked immunosorbent assays (ELISA) or lateral flow immunochromatographic assays are simple and rapid biochemical immunoassays, but they have a low sensitivity [
9,
10]. Similarly, polymerase chain reaction (PCR), biochips, and microarrays are some, but not all, of the nucleic-acid-based techniques that have been used for the investigation of foodborne microbes [
6,
9,
11]. Nevertheless, various types of PCR techniques such as reverse transcriptase and multiplex PCR are ineffective at processing a large volume of samples without a pre-enrichment step and have high processing costs that make them impractical for day-to-day use [
12].
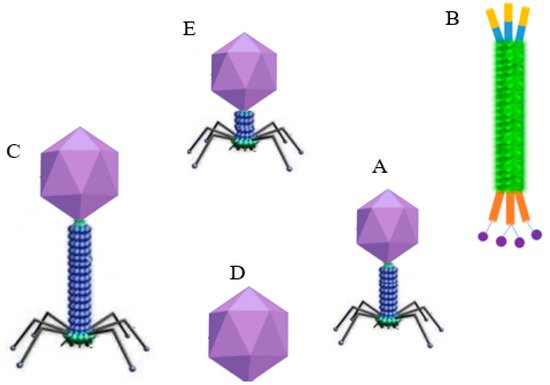
Over the last few decades, bacteriophage-based biosensors have been recognized as a promising platform for detecting pathogens or sensing various biological analytes. Compared to other bio-receptors such as aptamers and antibodies, bacteriophages provide quite a few advantages in the detection of pathogens. Firstly, phages have a unique structure, including tail fibers that aid their binding to bacterial hosts, are highly specific, and are harmless to human cells (
Figure 1). Virulent phages take 1–2 h to complete the infection cycle, quickening the release of the cytoplasmic marker from the infected host to be used in numerous detection systems. In addition, phages are the most abundant biological entities and are found in places where their host organism exists. They are relatively stable under various conditions, such as pH, temperatures, and organic/inorganic solvents, and they resist proteases. They are also cheaper to produce than antibodies and have a relatively long shelf life. It is easier to distinguish dead from live bacterial cells using this platform, as phages replicate only inside living bacteria [
12].
Figure 1. Schematic diagram showing the structure of major phage families: (A) Myoviridae (e.g., T4); (B) filamentous Inoviridae (e.g., M13); (C) long and noncontractile Siphoviridae (e.g., λ phage); (D) Leviviridae; and (E) short Podoviridae (e.g., T7).
The short shelf life of food products and the low infectious dose of most foodborne pathogens [
13] are the most critical driving forces that push researchers to design sensitive, specific, and reliable detection techniques. The development of phage-based biosensors as a tool for the direct detection of live pathogens in food is an important and attractive approach [
14]. Presently, several phage-based biosensors have been developed that incorporate various transducers, including electrochemical [
15], quartz crystal microbalance (QCM) [
16], surface plasmon resonance (SPR) [
17], magnetoelastic (ME) [
18], and others. Most of these biosensors have been designed using the whole/intact phage or the phage proteins as well as the cytoplasmic markers that are released following the phage infection. The performance of these biosensors varies, as they employ different immobilization methodologies (physical, chemical, covalent, or oriented) and/or transducers.
2. Phage-Based Biosensors
According to the International Union of Pure and Applied Chemistry (IUPAC), the biosensor is defined as a self-controlled derivative material that contains a bio-recognition component (bio-receptor/bio-probe) linked to a transducer (sensor) to convert the biological signal into a digital signal in the computer system for interpretation [
19]. Phage-based biosensor platforms generally consist of the network of the whole phage or partial phage particle, infection of the host bacterium, and finally production of colorimetric, electrical, fluorescent, or luminescent signals [
20,
21,
22].
Lytic bacteriophages are primarily classified under the order
Caudovirales (
Figure 1) and are the principal biorecognition entities used as probes for phage-based biosensors. Apart from lytic phages, temperate phages also play a comparable role in the development of phage-based biosensors. Both lytic and temperate phages, such as HK620, P22, and ΦV10, have been used to develop reporter (engineered) phages [
14]. Reporter phages are genetically modified by incorporating a reporter gene sequence into the phage genome to generate a measurable signal inside the intact host cell without killing (lysing) the host cell for the detection of live pathogens [
14]. Moreover, proteins such as phage receptor-binding proteins (RBPs) have been recognized to be efficient bio-probes for replacing antibodies or other biomolecules, and have been used in the design of various types of biosensors [
23]. In comparison to the whole phages, RBPs provide better stability across a broad range of pH values, temperatures, and gastrointestinal proteases [
24]. Remarkably, appropriate tags (amino acids, e.g., cysteine) can be added to the RBP sequence at a specific site without affecting the binding ability and can be employed for the oriented surface functionalization of the RBPs on the biosensor platforms [
24].
Bacteriophage-based biosensors offer several benefits for rapid bacterial detection [
25]. They are highly specific towards their host organism, resist high temperatures (90–97 °C), and are stable across a wide range of pH values (3–14) and organic solvents. In comparison to antibodies, phages can be produced in large quantities easily and cheaply. They are eco-friendly and safe to use since they do not infect humans [
26]. These characteristics make phages a novel bio-recognition tool for the development of biosensors for the detection of foodborne bacterial pathogens [
27,
28].
Today, phage-mediated biosensors have been developed as novel diagnostic tools in which specific phages are fixed to the device’s surface and then enabled to detect the analyte found in the sample [
29]. Bacteriophages can be immobilized on a solid material with the aid of chemical, physical, or other immobilization or tethering techniques. The capture of targeted bacterial cells by surface-immobilized virions is an event that ends up with specific detection. The detection of pathogens using phage-based sensors is not limited to clinical samples, but is also used in a wide range of nonclinical applications, including foodborne pathogens from water and various food matrices [
30], such as milk [
31] and other perishable and non-perishable foodstuffs [
32].
This entry is adapted from the peer-reviewed paper 10.3390/bios12100905