It has been estimated that between 30 and 50 per cent of all injuries that take place throughout participation in a sport are the consequence of soft tissue injuries, and muscle injuries are the primary cause of physical disability. Due to the produced effects, manual therapy combined with high-intensity laser therapy (Hilt) or capacitive and resistive electrical transfer (TECAR) therapy, or even all three therapies used in a treatment plan, can provide optimal muscle recovery in a shorter time; therefore, the use of combined and not isolated recovery procedures is a better alternative in muscle recovery.
- TECAR therapy
- high-intensity laser therapy
- manual therapy
1. Manual Therapy vs. TECAR vs. HILT Therapy
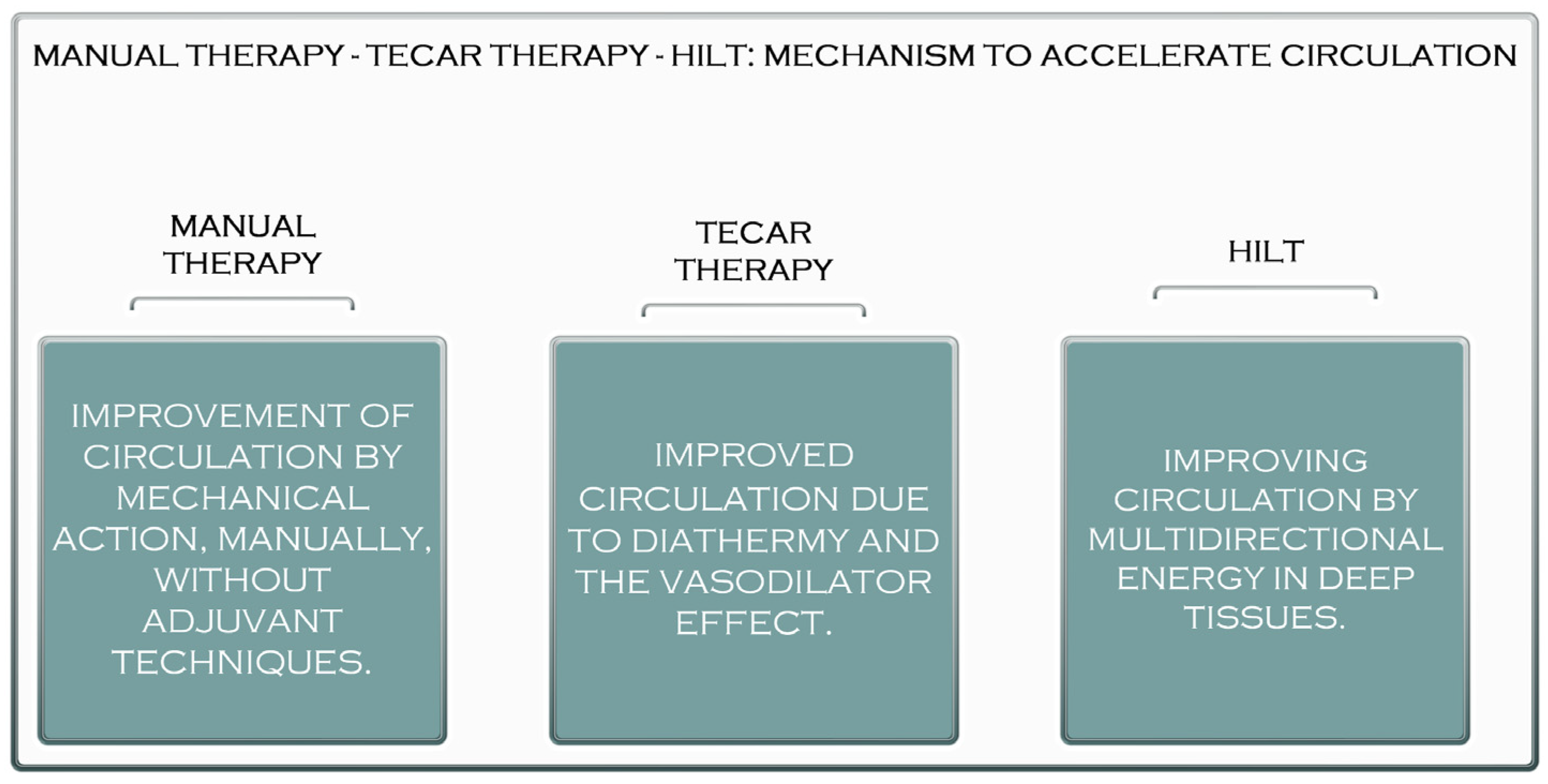
2. The Benefits of Manual Therapy in the Treatment of Muscle Diseases
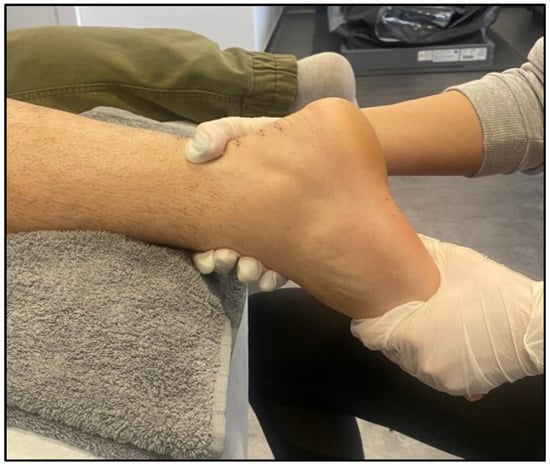
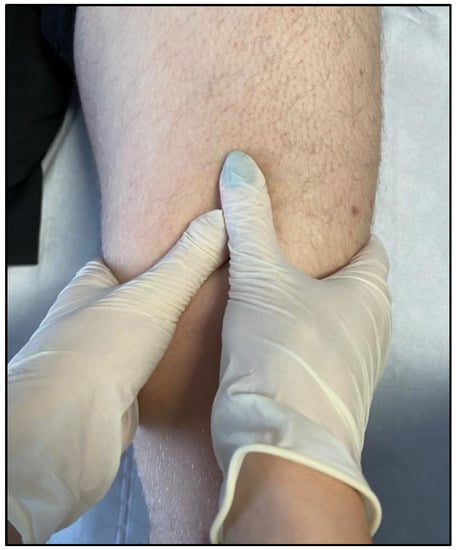
3. The Benefits of HILT Therapy in the Treatment of Muscle Disorders
- Thyroid disorders: The thyroid is known to be sensitive to light, and although it has not yet been found to harm the thyroid gland, caution and careful dosing are recommended when using the laser [42].
- Clotting problems: Laser therapy affects blood clotting, so its use for patients with such problems should be consulted with a specialist or even replaced with another procedure [43].
- Children: Although there is no contraindication to the use of the laser for children, its dose should be adjusted according to weight [44].
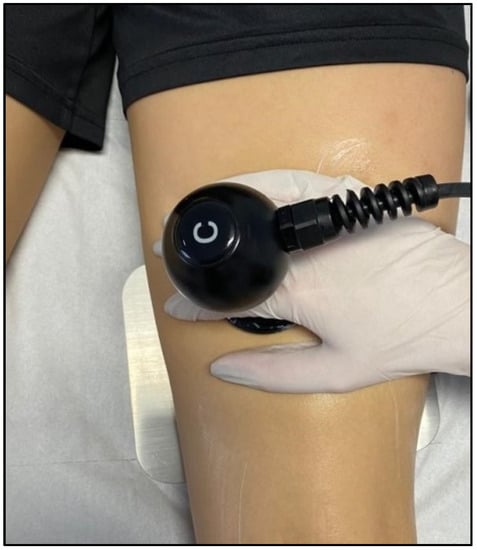
4. The Benefits of TECAR Therapy in the Treatment of Muscle Diseases
This entry is adapted from the peer-reviewed paper 10.3390/jcm11206149
References
- Elias, G.P.; Varley, M.C.; Wyckelsma, V.L.; McKenna, M.J.; Minahan, C.L.; Aughey, R.J. Effects of water immersion on post training recovery in Australian footballers. Int. J. Sports Physiol. Perform. 2012, 7, 357–366.
- Leeder, J.; Gissane, C.; van Someren, K.; Gregson, W.; Howatson, G. Cold water immersion and recovery from strenuous exercise: A meta-analysis. Br. J. Sports Med. 2012, 46, 233–240.
- Osti, R.; Pari, C.; Salvatori, G.; Massari, L. Tri-length laser therapy associated to TECAR therapy in the treatment of low-back pain in adults: A preliminary report of a prospective case series. Lasers Med. Sci. 2015, 30, 407–412.
- Pereira, W.M.; Ferreira, L.A.; Rossi, L.P.; Kerpers, I.I.; Grecco, S.L.A.; de Paula, A.R.J.; Oliveira, C.S. Influence of heat on fatigue and electromyographic activity of the biceps brachii muscle. J. Bodyw. Mov. Ther. 2011, 15, 478–484.
- Hayashi, K.; Arai, Y.C.; Ikemoto, T.; Nishihara, M.; Suzuki, S.; Hirakawa, T.; Matsuo, S.; Kobayashi, M.; Haruta, M.; Kawabata, Y.; et al. Predictive factors for the outcome of multidisciplinary treatments in chronic low back pain at the first multidisciplinary pain center of Japan. J. Phys. Ther. Sci. 2015, 27, 2901–2905.
- Alayat, M.S.; Atya, A.M.; Ali, M.M.; Shosha, T.M. Long-term effect of high-intensity laser therapy in the treatment of patients with chronic low back pain: A randomised blinded placebo-controlled trial. Lasers Med. Sci. 2014, 29, 1065–1073.
- Pillastrini, P.; Gardenghi, I.; Bonetti, F.; Capra, F.; Guccione, A.; Mugnai, R.; Violante, F.S. An updated overview of clinical guidelines for chronic low back pain management in primary care. Jt. Bone Spine 2012, 79, 176–185.
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell Physiol. Biochem. 2014, 34, 1741–1755.
- Kato, S.; Saitoh, Y.; Miwa, N. Repressive effects of a capacitive-resistive electric transfer (CRet) hyperthermic apparatus combined with provitamin C on intracellular lipid-droplets formation in adipocytes. Int. J. Hyperth. 2013, 29, 30–37.
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical Stimulation Promotes Stem Cell Neural Differentiation in Tissue Engineering. Stem Cells Int. 2021, 2021, 6697574.
- Oostendorp, R.A.B. Credibility of manual therapy is at stake “Where do we go from here?”. J. Man Manip. Ther. 2018, 26, 189–192.
- Reid, D.; Cook, C.; Sizer, P.S. Is orthopedic manipulative physical therapy not fashionable anymore? Lessons learned from 2016 IFOMPT meeting and future directions. J. Man Manip. Ther. 2017, 25, 1–2.
- Babatunde, O.O.; Jordan, J.L.; Van der Windt, D.A.; Hill, J.C.; Foster, N.E.; Protheroe, J. Effective treatment options for musculoskeletal pain in primary care: A systematic overview of current evidence. PLoS ONE 2017, 12, e0178621.
- Kim, G.J.; Choi, J.; Lee, S.; Jeon, C.; Lee, K. The effects of high intensity laser therapy on pain and function in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2016, 28, 3197–3199.
- Conforti, M.; Fachinetti, G.P. High power laser therapy treatment compared to simple segmental physical rehabilitation in whiplash injuries (1° and 2° grade of the Quebec Task Force classification) involving muscles and ligaments. Muscles Ligaments Tendons J. 2013, 3, 106–111.
- Ezzati, K.; Fekrazad, R.; Raoufi, Z. The effects of photo-biomodulation therapy on post-surgical pain. J. Lasers Med. Sci. 2019, 10, 79–85.
- Thabet, A.A.E.; Elsodany, A.M.; Battecha, K.H.; Alshehri, M.A.; Refaat, B. High-intensity laser therapy versus pulsed electromagnetic field in the treatment of primary dysmenorrhea. J. Phys. Ther. Sci. 2017, 29, 1742–1748.
- Thabet, A.A.E.; Mahran, H.G.; Ebid, A.A.; Alshehri, M.A. Effect of pulsed high intensity laser therapy on delayed caesarean section healing in diabetic women. J. Phys. Ther. Sci. 2018, 30, 570–575.
- Pekyavas, N.O.; Baltaci, G. Short-term effects of high-intensity laser therapy, manual therapy, and Kinesio taping in patients with subacromial impingement syndrome. Lasers Med. Sci. 2016, 31, 1133–1141.
- Ordahan, B.; Karahan, A.Y.; Kaydok, E. The effect of high-intensity versus low-level laser therapy in the management of plantar fasciitis: A randomised clinical trial. Lasers Med. Sci. 2018, 33, 1363–1369.
- Taradaj, J.; Rajfur, K.; Shay, B.; Rajfur, J.; Ptaszkowski, K.; Walewicz, K.; Dymarek, R.; Sopel, M.; Rosińczuk, J. Photo-biomodulation using high—Or low-level laser irradiations in patients with lumbar disc degenerative changes: Disappointing outcomes and remarks. Clin. Interv. Aging 2018, 13, 1445–1455.
- Song, H.J.; Seo, H.J.; Lee, Y.; Kim, S.K. Effectiveness of high-intensity laser therapy in the treatment of musculoskeletal disorders: A systematic review and meta-analysis of randomised controlled trials. Medicine 2018, 97, e13126.
- Thoomes, E.J.; Scholten-Peeters, W.; Koes, B.; Falla, D.; Verhagen, A.P.; Clin, J. The effectiveness of conservative treatment for patients with cervical radiculopathy: A systematic review. Pain 2013, 29, 1073–1086.
- Kheshie, A.R.; Alayat, M.S.; Ali, M.M. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomised controlled trial. Lasers Med. Sci. 2014, 29, 1371–1376.
- Yıldırıım, M.A.; Uçar, D.; Öneş, K. Comparison of therapeutic duration of therapeutic ultrasound in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 3667–3670.
- Boyraz, I.; Yildiz, A.; Koc, B.; Sarman, H. Comparison of high-intensity laser therapy and ultrasound treatment in the patients with lumbar discopathy. Biomed. Res. Int. 2015, 2015, 304328.
- Zielińska, P.; Nicpoń, J.; Kiełbowicz, Z.; Soroko, M.; Dudek, K.; Zaborski, D. Effects of high intensity laser therapy in the treatment of tendon and ligament injuries in performance horses. Animals 2020, 10, 1327.
- Ezzati, K.; Laakso, E.L.; Salari, A.; Hasannejad, A.; Fekrazad, R.; Aris, A. The beneficial effects of high-intensity laser therapy and co-interventions on musculoskeletal pain management: A systematic review. J. Lasers Med. Sci. 2020, 11, 81–90.
- Abdelbasset, W.K.; Nambi, G.; Alsubaie, S.F.; Abodonya, A.M.; Saleh, A.K.; Ataalla, N.N.; Ibrahim, A.A.; Tantawy, S.A.; Kamel, D.M.; Verma, A.; et al. A randomised comparative study between high-intensity and low-level laser therapy in the treatment of chronic nonspecific low back pain. Evid. Based Complement. Altern. Med. 2020, 2020, 1350281.
- Pellegrino, R.; Paolucci, T.; Brindisino, F.; Mondardini, P.; Di Iorio, A.; Moretti, A.; Iolascon, G. Effectiveness of High-Intensity Laser Therapy Plus Ultrasound-Guided Peritendinous Hyaluronic Acid Compared to Therapeutic Exercise for Patients with Lateral Elbow Tendinopathy. J. Clin. Med. 2022, 11, 5492.
- Alayat, M.S.; El Soudany, A.M.; El Fiky, A.A. Efficacy of high—And low-level laser therapy in the treatment of Bell’s palsy: A randomised double-blind placebo-controlled trial. Lasers Med. Sci. 2014, 29, 335–342.
- Alayat, M.S.; El Soudany, A.M.; Ali, M.E. Efficacy of multiwave locked system laser on pain and function in patients with chronic neck pain: A randomised placebo-controlled trial. Photomed. Laser Surg. 2017, 35, 450–455.
- Alayat, M.S.; Aly, T.H.A.; Elsayed, A.E.M.; Fadil, A.S.M. Efficacy of pulsed Nd:YAG laser in the treatment of patients with knee osteoarthritis: A randomised controlled trial. Lasers Med. Sci. 2017, 32, 503–511.
- Thabet, A.A.E.; Alshehri, M.A. Effect of pulsed high-intensity laser therapy on pain, adhesions, and quality of life in women having endometriosis: A randomized controlled trial. Photomed. Laser Surg. 2018, 36, 363–369.
- Tkocz, P.; Matusz, T.; Kosowski, Ł.; Walewicz, K.; Argier, Ł.; Kuszewski, M.; Hagner-Derengowska, M.; Ptaszkowski, K.; Dymarek, R.; Taradaj, J. A Randomised-Controlled Clinical Study Examining the Effect of High-Intensity Laser Therapy (HILT) on the Management of Painful Calcaneal Spur with Plantar Fasciitis. J. Clin. Med. 2021, 10, 4891.
- Alayat, M.S.M.; Abdel-Kafy, E.M.; Elsoudany, A.M.; Helal, O.F.; Alshehri, M.A. Efficacy of high intensity laser therapy in the treatment of male with osteopenia or osteoporosis: A randomised placebo-controlled trial. J. Phys. Ther. Sci. 2017, 29, 1675–1679.
- Ebid, A.A.; El-Kafy, E.M.; Alayat, M.S. Effect of pulsed Nd:YAG laser in the treatment of neuropathic foot ulcers in children with spina bifida: A randomised controlled study. Photomed. Laser Surg. 2013, 31, 565–570.
- Wyszynska, J.; Bal-Bochenska, M. Efficacy of high-intensity laser therapy in treating knee osteoarthritis: A first systematic review. Photomed. Laser Surg. 2018, 36, 343–353.
- Cotler, H.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The use of low-level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015, 2, 00068.
- Tortorici, S.; Messina, P.; Scardina, G.A. Effectiveness of low-level laser therapy on pain intensity after lower third molar extraction. Int. J. Clin. Dent. 2019, 12, 357–367.
- Chow, R.; Armati, P.; Laakso, E.L.; Bjordal, J.M.; Baxter, G.D. Inhibitory effects of laser irradiation on peripheral mammalian nerves and relevance to analgesic effects: A systematic review. Photomed. Laser Surg. 2011, 29, 365–381.
- Holanda, V.M.; Chavantes, M.C.; Wu, X.; Anders, J.J. The mechanistic basis for photo-biomodulation therapy of neuropathic pain by near infrared laser light. Lasers Surg. Med. 2017, 49, 516–524.
- Ketz, A.K.; Byrnes, K.R.; Grunberg, N.E. Characterization of macrophage/microglial activation and effect of photo-biomodulation in the spared nerve injury model of neuropathic pain. Pain Med. 2017, 18, 932–946.
- Thabet, A.A.; Ebid, A.A.; El-Boshy, M.E.; Almuwallad, A.O.; Hudaimoor, E.A.; Alsaeedi, F.E.; Alsubhi, R.H.; Almatrook, R.H.; Aljifry, R.F.; Alotaibi, S.H.; et al. Pulsed high-intensity laser therapy versus low level laser therapy in the management of primary dysmenorrhea. J. Phys. Ther. Sci. 2021, 33, 695–699.
- Collins, C.K.; Masaracchio, M.; Brismée, J.-M. The future of orthopedic manual therapy: What are we missing? J. Man Manip. Ther. 2017, 25, 169–171.
- Coronado, R.A.; Bialosky, J.E. Manual physical therapy for chronic pain: The complex whole is greater than the sum of its parts. J. Man Manip. Ther. 2017, 25, 115–117.
- Duñabeitia, I.; Arrieta, H.; Torres-Unda, J.; Gil, J.; Santos-Concejero, J.; Gil, S.M.; Irazusta, J.; Bidaurrazaga-Letona, I. Effects of a capacitive-resistive electric transfer therapy on physiological and biomechanical parameters in recreational runners: A randomised controlled crossover trial. Phys. Ther. Sport 2018, 32, 227–234.
- Diego, I.M.A.; Fernández-Carnero, J.; Val, S.L.; Cano-de-la-Cuerda, R.; Calvo-Lobo, C.; Piédrola, R.M.; Oliva, L.C.L.; Rueda, F.M. Analgesic effects of a capacitive-resistive monopolar radiofrequency in patients with myofascial chronic neck pain: A pilot randomised controlled trial. Rev. Assoc. Med. Bras. 2019, 65, 156–164.
- Rodríguez-Sanz, J.; López-De-Celis, C.; Hidalgo-García, C.; Canet-Vintró, M.; Fanlo-Mazas, P.; Pérez-Bellmunt, A. Temperature and current flow effects of different electrode placement in shoulder capacitive-resistive electric transfer applications: A cadaveric study. BMC Musculoskelet. Disord. 2021, 22, 139.
- Sousa, L.D.S.-D.; Sanchez, C.T.; Maté-Muñoz, J.L.; Hernández-Lougedo, J.; Barba, M.; Lozano-Estevan, M.D.C.; Garnacho-Castaño, M.V.; García-Fernández, P. Application of Capacitive-Resistive Electric Transfer in Physiotherapeutic Clinical Practice and Sports. Int. J. Environ. Res. Public Health 2021, 18, 12446.
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and resistive electric transfer therapy in rehabilitation: A systematic review. Int. J. Rehabil. Res. 2020, 43, 291–298.
- Nakamura, M.; Sato, S.; Kiyono, R.; Yahata, K.; Yoshida, R.; Kasahara, K.; Konrad, A. The Effect of Capacitive and Resistive Electric Transfer Intervention on Delayed-Onset Muscle Soreness Induced by Eccentric Exercise. Int. J. Environ. Res. Public Health 2022, 19, 5723.
- López-De-Celis, C.; Hidalgo-García, C.; Pérez-Bellmunt, A.; Fanlo-Mazas, P.; González-Rueda, V.; Tricás-Moreno, J.M.; Ortiz, S.; Rodríguez-Sanz, J. Thermal and non-thermal effects off capacitive-resistive electric transfer application on the Achilles tendon and musculotendinous junction of the gastrocnemius muscle: A cadaveric study. BMC Musculoskelet. Disord. 2020, 21, 46.
- Ostrowski, J.; Herb, C.C.; Scifers, J.; Gonzalez, T.; Jennings, A.; Breton, D. Comparison of muscle temperature increases produced by moist hot pack and ThermoStim probe. J. Sport Rehabil. 2019, 28, 459–463.
- Martínez-Pizarro, S. Transferencia eléctrica capacitiva y resistiva para mitigar el dolor . Rehabilitacion 2020, 54, 221–222.
- Tashiro, Y.; Hasegawa, S.; Yokota, Y.; Nishiguchi, S.; Fukutani, N.; Shirooka, H.; Tasaka, S.; Matsushita, T.; Matsubara, K.; Nakayama, Y.; et al. Effect of capacitive and resistive electric transfer on haemoglobin saturation and tissue temperature. Int. J. Hyperth. 2017, 33, 696–702.
- Hernández-Bule, M.L.; Trillo, M.Á.; Úbeda, A. Molecular mechanisms underlying antiproliferative and differentiating responses of hepatocarcinoma cells to subthermal electric stimulation. PLoS ONE 2014, 9, e84636.
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25.
- Paolucci, T.; Pezzi, L.; Centra, M.A.; Porreca, A.; Barbato, C.; Bellomo, R.G.; Saggini, R. Effects of capacitive and resistive electric transfer therapy in patients with painful shoulder impingement syndrome: A comparative study. J. Int. Med. Res. 2020, 48, 300060519883090.
- Bito, T.; Tashiro, Y.; Suzuki, Y.; Kajiwara, Y.; Zeidan, H.; Kawagoe, M.; Sonoda, T.; Nakayama, Y.; Yokota, Y.; Shimoura, K.; et al. Acute effects of capacitive and resistive electric transfer (CRet) on the Achilles tendon. Electromagn. Biol. Med. 2019, 38, 48–54.
- Wostyn, V. La Tecarthérapie Appliquée a la Kinésithérapie: Evaluation de Leffet Antalgique Immédiat; Institut de Formation on Masso-Kinésithérapie: Reims, France, 2015.
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the application of TECAR therapy affect temperature and perfusion of skin and muscle microcirculation? A pilot feasibility study on healthy subjects. J. Altern. Complement. Med. 2020, 26, 147–153.
- López-de-Celis, C.; Rodríguez-Sanz, J.; Hidalgo-García, C.; Cedeño-Bermúdez, S.A.; Zegarra-Chávez, D.; Fanlo-Mazas, P.; Pérez-Bellmunt, A. Thermal and Current Flow Effects of a Capacitive-Resistive Electric Transfer Application Protocol on Chronic Elbow Tendinopathy. A Cadaveric Study. Int. J. Environ. Res. Public Health 2021, 18, 1012.
- Masiero, S.; Pignataro, A.; Piran, G.; Duso, M.; Mimche, P.; Ermani, M.; Del Felice, A. Short-wave diathermy in the clinical management of musculoskeletal disorders: A pilot observational study. Int. J. Biometeorol. 2020, 64, 981–988.
- Ozen, S.; Doganci, E.B.; Ozyuvali, A.; Yalcin, A.P. Effectiveness of continuous versus pulsed short-wave diathermy in the management of knee osteoarthritis: A randomised pilot study. Casp. J. Intern Med. 2019, 10, 431–438.
- Koller, T. Mechanosensitive Aspects of Cell Biology in Manual Scar Therapy for Deep Dermal Defects. Int. J. Mol. Sci. 2020, 21, 2055.
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895.
- Priego-Quesada, J.I.; De la Fuente, C.; Kunzler, M.R.; Perez-Soriano, P.; Hervás-Marín, D.; Carpes, F.P. Relationship between Skin Temperature, Electrical Manifestations of Muscle Fatigue, and Exercise-Induced Delayed Onset Muscle Soreness for Dynamic Contractions: A Preliminary Study. Int. J. Environ. Res. Public Health 2020, 17, 6817.
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Covelli, I.; Tafuri, S.; Moretti, B. Short term efficacy of capacitive resistive diathermy therapy in patients with low back pain: A prospective randomised controlled trial. J. Biol. Regul. Homeost. Agents 2017, 31, 509–515.
- Yokota, Y.; Tashiro, Y.; Suzuki, Y. Effect of capacitive and resistive electric transfer on tissue temperature, muscle flexibility and blood circulation. J. Nov. Physiother. 2017, 30, 719–725.
- Niajalili, M.; Sedaghat, M.; Reazasoltani, A.; Akbarzade Baghban, A.R.; Naimi, S.S. Effect of Capacitive Tecar Therapy on Foot Pain and Tactile Sensation in Patients with Type 2 Diabetes. Arch. Rehab. 2020, 21, 304–319.
- Du, J.; Zhen, G.; Chen, H.; Zhang, S.; Qing, L.; Yang, X.; Lee, G.; Mao, H.Q.; Jia, X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018, 181, 347–359.
- Zaretsky, D.V.; Romanovsky, A.A.; Zaretskaia, M.V.; Molkov, Y.I. Tissue oxidative metabolism can increase the difference between local temperature and arterial blood temperature by up to 1.3 °C: Implications for brain, brown adipose tissue, and muscle physiology. Temperature 2018, 5, 22–35.
