Sphingosine kinase (SPHK) catalyses the ATP-dependent phosphorylation of sphingosine to form sphingosine 1-phosphate (S1P), which acts as an intracellular second messenger and extracellular ligand for specific receptors. S1P can be released through specific transporters to act as a ligand for the family of G protein-coupled S1P receptors 1 to 5 (S1P1 to S1P5) and regulates a wide range of biological effects including transformation and cancer cell survival. S1P levels are tightly regulated by the balance between synthesis by SPHK, reversible conversion to sphingosine by specific S1P phosphatases (SPP1 and SPP2), and degradation by S1P lyase.
- cancer stem cells
- sphingosine kinase
- STAT1
- mammospheres
- drug synergism
- sphingolipids
1. Introduction
Breast cancer stem cells (CSCs) represent a subset of cancer cells with the capabilities of self-renewal and differentiation [1] . Although several signaling pathways (such as STAT3 [2], Wnt/β-Catenin[3], Notch [4], Hedgehog [5], and NFκB [6]) have been implicated in regulating the growth and survival of breast CSCs, designing selective CSC-targeted strategies using these pathways remains a challenge as these pathways also share common functional roles in the maintenance of normal stem cells [7].
2. Datas
In contrast to S1P, which is associated with growth and survival, its precursors, sphingosine and ceramide, are associated with cell growth arrest and apoptosis [11]. According to the sphingolipid rheostat model, the balance between these interconvertible sphingolipids, ceramide, sphingosine, and S1P, regulates cellular growth and survival in response to cellular and environmental stimuli [12][13]. Thus, SPHK is a critical regulator of this rheostat, as it produces the pro-growth and anti-apoptotic S1P and also reduces levels of pro-apoptotic ceramide and sphingosine [11][14][15][16][17][18]. Thus, the inhibition of SPHK is likely to have an anti-cancer effect by producing apoptotic ceramide/sphingosine.
There are two isoforms of sphingosine kinase called SPHK1 and SPHK2. Several studies have indicated that increased SPHK1 activity promotes cancer cell growth, metastasis, and inhibits apoptosis [9][10][11][19][20][21]. Indeed, high expression of SPHK1 in tumors is associated with worse prognosis and overall outcomes in breast cancer patients [21][22][23][24][25][26][27][28]. In addition, overexpression of SPHK1 in breast cancer cells was reported to increase breast CSCs and the tumorigenicity of tumors in nude mice via S1P binding to S1P3 and down-stream stimulation of Notch and p38 MAPK signaling [29]. Furthermore, benzyl butyl phthalate, a carcinogen that has been shown to induce SPHK1 expression through activation of the aryl hydrocarbon receptor (AhR), was recently shown to enhance the formation of metastasis-initiating breast CSCs, suggesting a role of SPHK1 in breast CSCs [30].
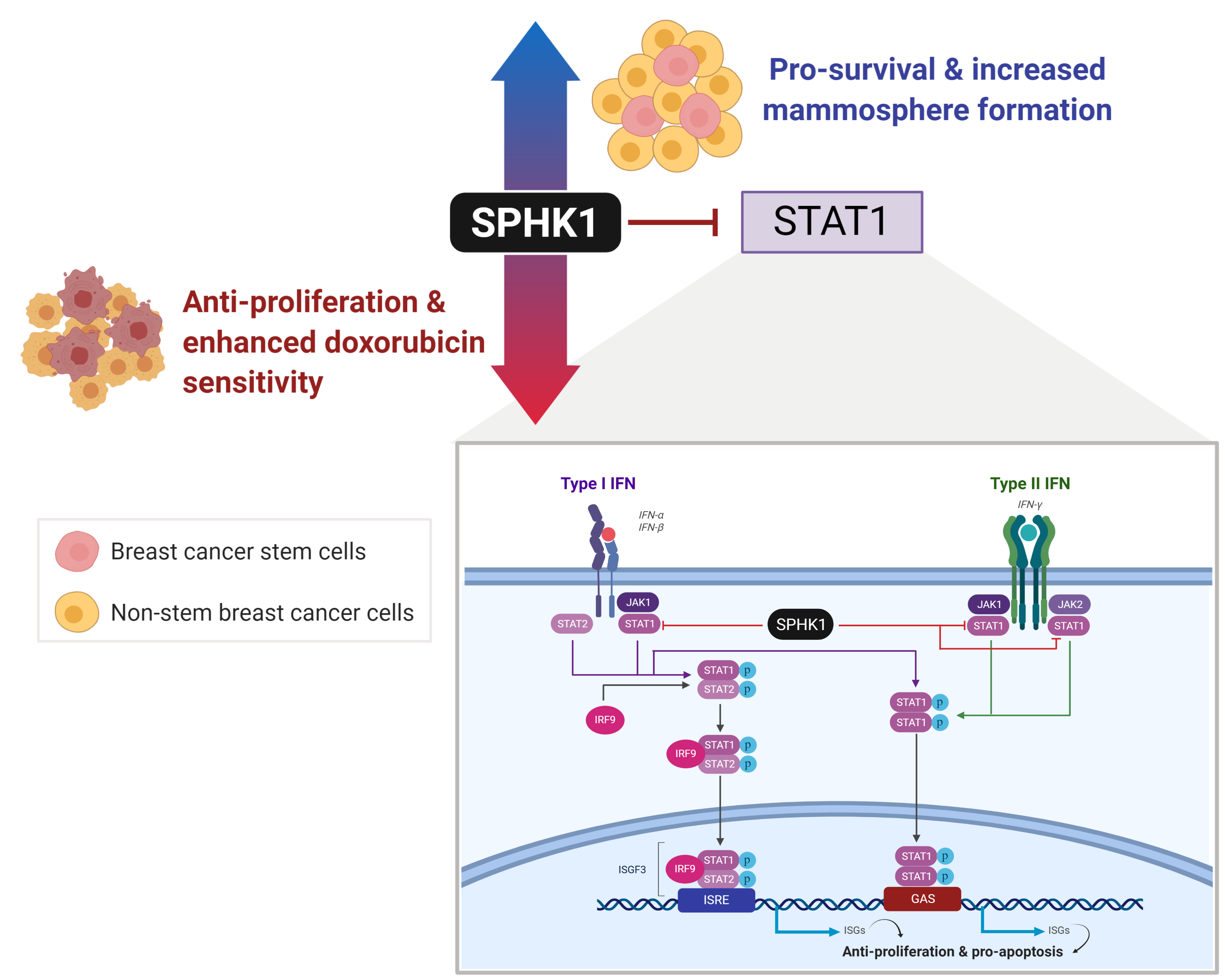
Using a kinome-wide shRNA library screen, we previously identified that SPHK1 is required for breast cancer cell survival [31]. However, whether SPHK1 is required for the survival of breast CSCs remains unknown. Hence, this study sought to investigate whether SPHK1 regulates the survival of breast CSCs, the underlying mechanism of this protection, and whether there are any substantive differences with its role in non-CSCs. In this regard, we demonstrate herein that SPHK1 expression is increased in breast CSCs compared with non-CSCs and is involved in regulating the survival of breast CSCs and non-CSCs through repression of the tumor suppressor function of STAT1 [32]. Importantly, selective inhibition of SPHK1 enhances doxorubicin sensitivity in breast CSCs and non-CSCs [32]. Overall, our results implicate SPHK1 as a potential target for the treatment of refractory breast cancers by targeting both breast CSCs and non-CSCs [32].
This entry is adapted from the peer-reviewed paper 10.3390/cells9040886
References
- Eduard Batlle; Hans Clevers; Cancer stem cells revisited. Nature Medicine 2017, 23, 1124-1134, 10.1038/nm.4409.
- Lauren L.C. Marotta; Vanessa Almendro; Andriy Marusyk; Michail Shipitsin; Janina Schemme; Sarah R. Walker; Noga Bloushtain-Qimron; Jessica J. Kim; Sibgat A. Choudhury; Reo Maruyama; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44⁺CD24⁻ stem cell-like breast cancer cells in human tumors.. Journal of Clinical Investigation 2011, 121, 2723-35, 10.1172/JCI44745.
- Felipe De Sousa E Melo; Louis Vermeulen; Wnt Signaling in Cancer Stem Cell Biology. Cancers 2016, 8, 60, 10.3390/cancers8070060.
- Gillian Farnie; Robert Clarke; Mammary Stem Cells and Breast Cancer—Role of Notch Signalling. Stem Cell Reviews and Reports 2007, 3, 169-175, 10.1007/s12015-007-0023-5.
- Ita Novita Sari; Lan Thi Hanh Phi; Nayoung Jun; Yoseph Toni Wijaya; Sanghyun Lee; Hyog Young Kwon; Hedgehog Signaling in Cancer: A Prospective Therapeutic Target for Eradicating Cancer Stem Cells. Cells 2018, 7, 208, 10.3390/cells7110208.
- Kateryna Shostak; Alain Chariot; NF-κB, stem cells and breast cancer: the links get stronger.. Breast Cancer Research 2011, 13, 214-214, 10.1186/bcr2886.
- Luisa Barbato; Marco Bocchetti; Anna Di Biase; Tarik Regad; Cancer Stem Cells and Targeting Strategies. Cells 2019, 8, 926, 10.3390/cells8080926.
- Susan Pyne; Melissa McNaughton; Stephanie Boomkamp; Neil MacRitchie; Cecilia Evangelisti; Alberto Martelli; Hui-Rong Jiang; Satvir Ubhi; Susan Pyne; Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Advances in Biological Regulation 2016, 60, 151-159, 10.1016/j.jbior.2015.09.001.
- Susan Pyne; Ashref El Buri; David R. Adams; Susan Pyne; Sphingosine 1-phosphate and cancer. Advances in Biological Regulation 2018, 68, 97-106, 10.1016/j.jbior.2017.09.006.
- Susan Pyne; Susan Pyne; Translational aspects of sphingosine 1-phosphate biology. Trends in Molecular Medicine 2011, 17, 463-472, 10.1016/j.molmed.2011.03.002.
- Susan Pyne; David R. Adams; Susan Pyne; Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Progress in Lipid Research 2016, 62, 93-106, 10.1016/j.plipres.2016.03.001.
- Zijian Fang; Susan Pyne; Susan Pyne; Ceramide and sphingosine 1-phosphate in adipose dysfunction.. Progress in Lipid Research 2019, 74, 145-159, 10.1016/j.plipres.2019.04.001.
- Susan Pyne; Jan Ohotski; Robert Bittman; Susan Pyne; The role of sphingosine 1-phosphate in inflammation and cancer. Advances in Biological Regulation 2014, 54, 121-129, 10.1016/j.jbior.2013.08.005.
- Susan Pyne; Susan Pyne; Sphingosine 1-phosphate is a missing link between chronic inflammation and colon cancer.. Cancer Cell 2013, 23, 5-7, 10.1016/j.ccr.2012.12.005.
- Susan Pyne; Francesca Tonelli; Keng Gat Lim; Jaclyn Long; Joanne Edwards; Susan Pyne; Targeting sphingosine kinase 1 in cancer. Advances in Biological Regulation 2012, 52, 31-38, 10.1016/j.advenzreg.2011.07.001.
- Susan Pyne; Robert Bittman; Susan Pyne; Sphingosine kinase inhibitors and cancer: seeking the golden sword of Hercules.. Cancer Research 2011, 71, 6576-82, 10.1158/0008-5472.CAN-11-2364.
- Susan Pyne; Joanne Edwards; Jan Ohotski; Nigel J. Pyne; Sphingosine 1-phosphate receptors and sphingosine kinase 1: novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Frontiers in Oncology 2012, 2, 168, 10.3389/fonc.2012.00168.
- Susan Pyne; Francesca Tonelli; Keng Gat Lim; Jaclyn S. Long; Joanne Edwards; Susan Pyne; Sphingosine 1-phosphate signalling in cancer. Biochemical Society Transactions 2012, 40, 94-100, 10.1042/bst20110602.
- Sunil Acharya; Jun Yao; Ping Li; Chenyu Zhang; Frank J. Lowery; Qingling Zhang; Hua Guo; Jingkun Qu; Fei Yang; Ignacio I. Wistuba; et al. Sphingosine Kinase 1 Signaling Promotes Metastasis of Triple-Negative Breast Cancer.. Cancer Research 2019, 79, 4211-4226, 10.1158/0008-5472.CAN-18-3803.
- Susan Pyne; Susan Pyne; Sphingosine 1-phosphate and cancer. Nature Reviews Cancer 2010, 10, 489-503, 10.1038/nrc2875.
- Jaclyn S. Long; Joanne Edwards; Carol Watson; Sian Tovey; Kirsty M. Mair; Rachel Schiff; Viswanathan Natarajan; Susan Pyne; Susan Pyne; Sphingosine Kinase 1 Induces Tolerance to Human Epidermal Growth Factor Receptor 2 and Prevents Formation of a Migratory Phenotype in Response to Sphingosine 1-Phosphate in Estrogen Receptor-Positive Breast Cancer Cells. Molecular and Cellular Biology 2010, 30, 3827-3841, 10.1128/mcb.01133-09.
- Arpita Datta; Ser Yue Loo; Baohua Huang; Lingkai Wong; Sheryl S.L. Tan; Tuan Zea Tan; Soo-Chin Lee; Jean Paul Thiery; Yaw Chyn Lim; Wei Peng Yong; et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget 2014, 5, 5920-5933, 10.18632/oncotarget.1874.
- Eugen Ruckhäberle; Achim Rody; Knut Engels; Regine Gaetje; Gunter Von Minckwitz; Susanne Schiffmann; Sabine Grösch; Gerd Geisslinger; Uwe Holtrich; Thomas Karn; et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Research and Treatment 2007, 112, 41-52, 10.1007/s10549-007-9836-9.
- Ya-Jing Zhu; Hua You; Jin-Xiang Tan; Fan Li; Zhu Qiu; Hong-Zhong Li; Hong-Yan Huang; Ke Zheng; Guo-Sheng Ren; Overexpression of sphingosine kinase 1 is predictive of poor prognosis in human breast cancer. Oncology Letters 2017, 14, 63-72, 10.3892/ol.2017.6134.
- Aparna Maiti; Kazuaki Takabe; Nitai C. Hait; Metastatic triple-negative breast cancer is dependent on SphKs/S1P signaling for growth and survival.. Cellular Signalling 2017, 32, 85-92, 10.1016/j.cellsig.2017.01.021.
- Carol Watson; Jaclyn S. Long; Clare Orange; Claire L. Tannahill; Elizabeth Mallon; Liane McGlynn; Susan Pyne; Susan Pyne; Joanne Edwards; High Expression of Sphingosine 1-Phosphate Receptors, S1P1 and S1P3, Sphingosine Kinase 1, and Extracellular Signal-Regulated Kinase-1/2 Is Associated with Development of Tamoxifen Resistance in Estrogen Receptor-Positive Breast Cancer Patients. The American Journal of Pathology 2010, 177, 2205-2215, 10.2353/ajpath.2010.100220.
- Jan Ohotski; Joanne Edwards; Beatrix Elsberge; Carol Watson; Clare Orange; Elizabeth Mallon; Susan Pyne; Nigel J Pyne; Identification of Novel Functional and Spatial Associations Between Sphingosine Kinase 1, Sphingosine 1-phosphate Receptors and Other Signaling Proteins That Affect Prognostic Outcome in Estrogen Receptor-Positive Breast Cancer. Int J Cancer 2013, 132(3), 605‐616, 10.1002/ijc.27692.
- J Ohotski; J S Long; C Orange; B Elsberger; E Mallon; J Doughty; Susan Pyne; Susan Pyne; Joanne Edwards; Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. British Journal of Cancer 2012, 106, 1453-1459, 10.1038/bjc.2012.98.
- Naoya Hirata; Shigeru Yamada; Takuji Shoda; Masaaki Kurihara; Yuko Sekino; Yasunari Kanda; Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nature Communications 2014, 5, 4806, 10.1038/ncomms5806.
- Yu-Chih Wang; Cheng-Fang Tsai; Hsiao-Li Chuang; Yi-Chih Chang; Hung-Sheng Chen; Jau-Nan Lee; Eing‑Mei Tsai; Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget 2016, 7, 29563-29576, 10.18632/oncotarget.9007.
- Kai Hung Tiong; Boon Shing Tan; Heng Lungh Choo; Felicia Fei-Lei Chung; Ling-Wei Hii; Si Hoey Tan; Nelson Tze Woei Khor; Shew Fung Wong; Sze-Jia See; Yuen-Fen Tan; et al. Fibroblast growth factor receptor 4 (FGFR4) and fibroblast growth factor 19 (FGF19) autocrine enhance breast cancer cells survival. Oncotarget 2016, 7, 57633-57650, 10.18632/oncotarget.9328.
- Ling-Wei Hii; Felicia Fei-Lei Chung; Chun Wai Mai; Zong Yang Yee; Hong Hao Chan; Vijay Joseph Raja; Noah Elias Dephoure; Susan Pyne; Susan Pyne; Chee-Onn Leong; et al. Sphingosine Kinase 1 Regulates the Survival of Breast Cancer Stem Cells and Non-stem Breast Cancer Cells by Suppression of STAT1. Cells 2020, 9, 886, 10.3390/cells9040886.