Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Composites can be derived from plant-based nanomaterials, among which is nanocellulose which has attracted significant attention as potential replacements for their more conventional petroleum-derived counterparts for use in chemical sensing applications.
- nanocellulose
- composites
- chemical sensors
- functionalization
1. Introduction
Recent developments in material science have seen a new class of materials known as “mixed materials” or “composites” emerging [1,2,3,4]. Polymer composite materials are materials that are made up of organic semiconductors and biomass derivatives that could be used in a variety of ways [5]. These combinations provide a material with improved mechanical strength, electrical conductivity and thermal stability [6,7,8]. Figure 1 illustrates composite materials used in a variety of different applications.
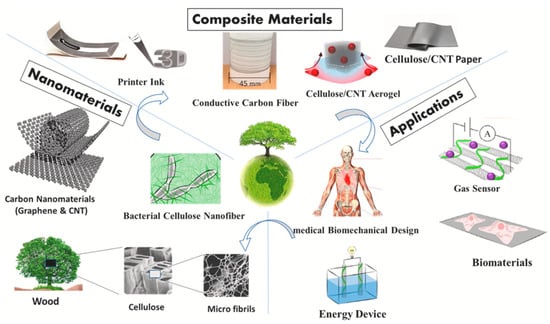
Figure 1. Polymer composite materials and its various applications. Adapted from Ref. [9].
Nowadays, polymer composites are being used more commonly in the development of chemical sensors [10,11,12,13]. Chemical sensors have gained significant attention in the various areas of public safety, such as in the military [14], space exploration [15], biomedicine [16], pharmaceuticals [17], leakage detection of explosive gases such as hydrogen [18], and real-time detection of toxic and carcinogenic gases in various industries [19,20], as well as chemical warfare agents [21,22], particularly at public venues such as airports and public parks. These chemical sensors are typically installed both indoors and outdoors, as well as used as portable devices to be brought into areas where the target analytes are suspected or spilled. The need for more accurate and sensitive chemical sensors has driven research and development of these sensors with the use of polymers and composites now being given more emphasis most likely due to the remarkable sensor performance resulting from their use. This improved sensor performance is increased sensitivity in the parts per million (ppm) to billion (ppb) range for trace level detection, absolute discrimination, and the sensors now becoming bio-based, reproducible, biocompatible, with the ability to operate at mild operational temperatures, having low power consumption, of a reasonable size, volume and mass, and with low cost for large-scale applications [23,24]. However, the development of the ideal chemical sensor is still far from realisation in spite of the enormous advances over the past few decades.
Recently, as various material science fields have expanded, there has been a surge in interest in further improving dependable graphene composite chemical sensors. Interestingly, nanomaterials are being actively investigated in the ongoing development of composites for chemical sensors. This is because of these nanomaterials having extraordinary physicochemical properties that are absent in their bulk form [25,26]. Hence, over the last few decades, nanomaterials have been actively investigated and then applied as core components of advantageous chemical sensing applications.
Composites can be derived from plant-based nanomaterials, among which is nanocellulose which has attracted significant attention as potential replacements for their more conventional petroleum-derived counterparts for use in chemical sensing applications. The current trend seen in publications related to this area show an extensive increase over this past decade. This is illustrated by a survey using Google Scholar using the keyword “nanocellulose composites for chemical sensors” which is shown in Figure 2. Nanocellulose possesses several interesting properties which includes being a renewable resource, having a large specific surface area, a porous structure, essentially biocompatible and with unique structural and physical characteristics such as tensile, optical and electrical properties which make it an ideal material for use in chemical sensors [14]. However, since the natural hydrophilic property of nanocellulose is not compatible with the hydrophobic nature of some sensing molecules, the development of suitable composites using surface functionalization on the nanocellulose is required to enable the needed sensor component compatibility.
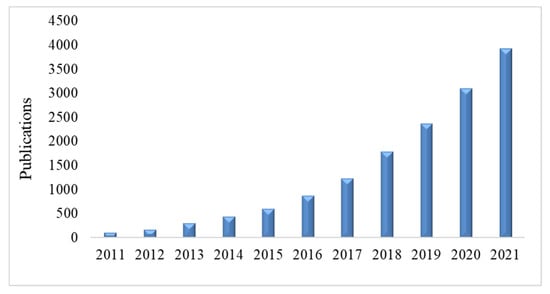
Figure 2. Total number of publications related to nanocellulose chemical sensors.
Nanocellulose composites are usually prepared by functionalizing them with a variety of conducting polymers such as polypyrrole (PPy), polyaniline (PANI) and poly (3,4-ethylenedioxythiophene) (PEDOT) derivatives [27,28,29,30]. This is to develop a sensor mediating material that has the necessary electronic characteristics together with the structural advantages that nanocellulose has. Conducting polymer nanostructures with large specific surface areas and a porous structure that are combined with suitable electrical properties have been reported to be excellent sensing mediators [31].
2. Nanocellulose’s Unique Characteristics as a Chemical Sensor
Natural cellulose constitutes the most abundant renewable polymer resource available worldwide [55,56,57]. As a raw material, it is generally well known for its use in the form of fibers or derivatives in a wide spectrum of products and materials for a multitude of uses. Cellulose fibrils are structural entities formed through a cellular manufacturing process, involving cellulose biogenesis, and stabilized by both hydrogen bonds and van der Waal forces. It contains an abundance of hydroxyl groups as shown in Figure 3. It can be further converted into nanocellulose through several pre-treatment approaches involving chemical, mechanical, physical, and enzymatical processes or through combination of processes thereof [58,59,60,61].
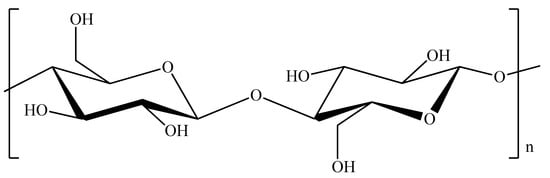
Figure 3. Chemical structure of cellulose.
Nanocellulose can be classified variously into cellulose nanocrystals (CNC), cellulose nanofibrils (CNF) and bacterial nanocellulose (BNC) depending on their production mode as well as their morphologies. CNC are long and straight crystals of cellulose with very large modulus elasticity and strength. CNC are needle-shaped crystalline fibrils that are 150–300 nm in length and 5–10 nm in diameter. They are mainly produced by the controlled acid hydrolysis of cellulose fibers which selectively dissolves the amorphous domains and releases cellulose crystallites. Meanwhile, CNF is composed of thin, flexible nanosized fibrils, encompassing both crystalline and amorphous domains. Their production involves mainly intensive mechanical disintegration. This is done using either high pressure homogenization, microfluidization or high-intensity mechanical grinding using wet disk milling [62,63,64,65]. On the other hand, BNC is made of cellulose nanofibrils which are obtained from certain types of bacteria using a bottom-up approach that involves the enzymatic polymerization of glucose. The diameter size for CNF and BNC are usually below than 100 nm [66]. The morphological properties of BNC are generally similar to those of CNF.
Nanocelluloses combine important cellulose properties such as hydrophilicity and crystalline characteristics, while containing a broad chemical modification capacity and a high surface area. The use of nanocellulose instead of other materials in composites is known to make certain products more efficient, biocompatible, cost-effective and environmentally friendly. Nanocellulose displays much merit thus making it a good material of choice for the development of chemical sensors.
Table 1 highlights major nanocellulose attributes that contribute to their use as chemical sensors.
Table 1. Merits of nanocellulose as chemical sensors.
| Properties | Description | References |
|---|---|---|
| Surface modification |
|
[67,68] |
| Nanocellulose structuring in solid films |
|
[69,70] |
| Large specific surface area/porous structure |
|
[71,72] |
| Tensile |
|
[73] |
| Optical/fluorescent |
|
[74,75] |
| Electrical |
|
[74] |
| Biodegradable |
|
[76] |
As was discussed above, nanocellulose in its native form has limitations in its application as a chemical sensor. Composites of nanocellulose for use as chemical sensors can be prepared through surface functionalization of the nanocellulose with other conducting polymers. A developed porous nanostructure conducting polymer with a large specific surface area that possesses electrical properties is predicted to be an excellent sensing mediator. The electrostatic interactions between the different charges found in nanocellulose composites and the analytes play an important role in the fabrication of ion exchange and permselective membranes. These developed nanocellulose composites can be modified by changing their surface functionality and permselective properties.
There are several different strategies of surface functionalization that can be used which involve the chemistry of hydroxyl functional groups found in nanocellulose as shown in Figure 5. Functionalization of nanocellulose can usually be carried out through several reactions such as covalent, oxidation, esterification and even more [77,78]. In order to covalently modify superficial porous nanocellulose, this would involve its treatment with strong acids, silylation, the addition of small functional groups, medium-sized molecules, macromolecules, polymers or even nanoparticles. Besides that, oxidants can also be used for tuning the surface (charges, functional groups) and their properties. TEMPO-mediated oxidation is the most employed reaction to enrich a nanocellulose surface with carboxylic acids. Functionalization of nanocellulose surfaces can also involve esterification reactions using a wide variety of modalities such as acetylation, acylation and cationization, which causes the surface of the nanocellulose to change dramatically through these reactions thereby causing them to possess more hydrophobic or cationic charges.
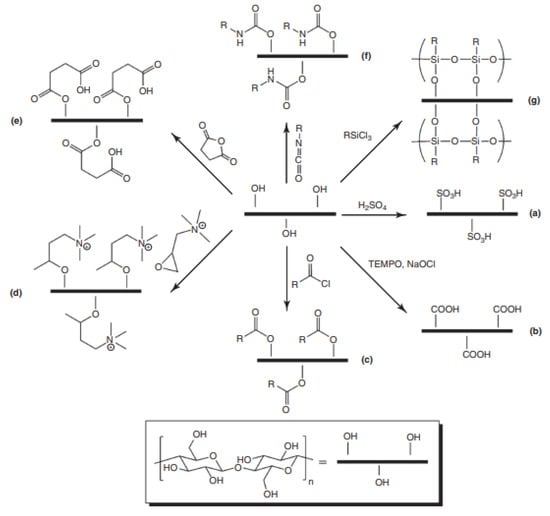
Figure 5. Some possible routes for chemical modification of nanocellulose: (a) sulfonation; (b) oxidation by TEMPO; (c) ester linkages via acid chlorides; (d) cationization via epoxides; (e) ester linkages via acid anhydrides; (f) urethane linkages via isocyanates; and (g) silylation. Adapted from Ref. [79].
Additionally, functionalization using other molecules onto nanocellulose surfaces has been extensively described [72,77,78,80,81]. Another very relevant example of surface modification involves polymer-grafting methods onto the nanocellulose. However, a common limitation using this reaction is when both polymer types are incompatible. This becomes a big challenge to increase the polar character of the nanocellulose to enable it to become compatible with other polymers. This incompatibility problem involving covalent linkages can be improved using several techniques. These include “grafting onto” which involves the attachment of an existing polymer onto nanocellulose using coupling agents which can be by employing a polymerization process that uses particular monomers and through the use of an initiator agent in the presence of the nanocellulose during processing. Beyond these, freeze-drying, hot-pressing and casting techniques have also been found to be suitable for increasing the compatibility of nanocellulose within polymeric matrices. However, the problem of nanocellulose agglomeration is that it then promotes sedimentation, which especially in a non-polar matrix continues to persist. Among methods to overcome this, melt extrusion techniques have been used to reduce this agglomeration.
This entry is adapted from the peer-reviewed paper 10.3390/polym14204461
This entry is offline, you can click here to edit this entry!
