Blood typing is a medical laboratory test used to identify the antigens on a person's red blood cells, which determine their blood type. Routine blood typing involves determining the ABO and RhD (Rh factor) type, and is performed before blood transfusions to ensure that the donor blood is compatible. It is also used to help diagnose hemolytic disease of the newborn, a condition caused by incompatibility between the blood types of a mother and her baby. ABO typing involves both identification of ABO antigens on red blood cells (forward grouping) and identification of ABO antibodies in the plasma (reverse grouping). Other blood group antigens, such as RhC/c and E/e or Kell, may be tested for in special situations. Blood typing is usually performed using serologic methods, which use reagents containing antibodies, called antisera, to identify blood group antigens. Serologic methods rely on the ability of antibodies to cause red blood cells to clump together when they bind to antigens on the cell surface, a phenomenon called agglutination. A number of serologic methods are available, ranging from manual blood typing using test tubes or slides to fully automated systems. Blood types can also be determined through genetic testing, which is used when conditions that interfere with serologic testing are present or when a high degree of accuracy in antigen identification is required. A number of conditions can cause false or inconclusive results in blood typing. When these issues affect ABO typing, they are called ABO discrepancies. ABO discrepancies must be investigated and resolved before the person's blood type is reported. There are different procedures for resolving ABO discrepancies depending on the underlying causes. Other sources of error in blood typing include the "weak D" phenomenon, in which people who are positive for the RhD antigen show weak or negative reactions when tested for RhD, and the presence of Immunoglobulin G antibodies on red blood cells, which interferes with typing for some blood group antigens.
- immunoglobulin
- red blood cells
- blood typing
1. Background
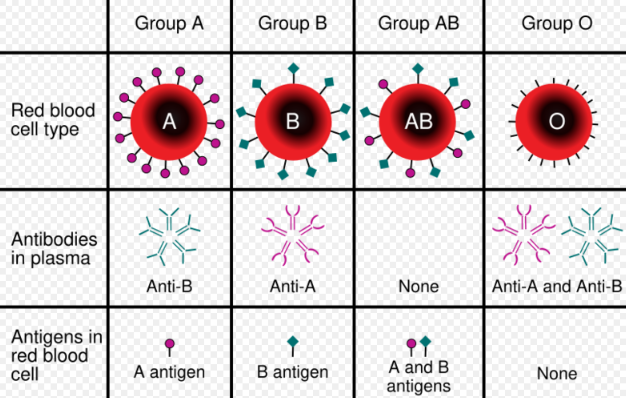
Blood types are defined according to the presence or absence of certain antigens on the surface of red blood cells. The most important of these in medicine are the ABO and RhD antigens[1] but many other blood group systems exist and are clinically relevant in certain situations. As of 2018, 346 human blood group antigens have been described, forming 36 different blood group systems.[2] Blood group antigens besides ABO and RhD that are significant in transfusion medicine include the RhC/c and E/e antigens and the antigens of the Duffy, Kell, Kidd, MNS, Lewis, I, P, and Lutheran systems.[2]
People who lack certain blood group antigens on their red cells can form antibodies against these antigens. For example, a person with type A blood will produce antibodies against the B antigen. The ABO blood group antibodies are naturally occurring, meaning that they are found in people who have not been exposed to incompatible blood.[1] Antibodies to most other blood group antigens, including RhD, develop after people are exposed to the antigens through transfusion or pregnancy.[2] Some of these antibodies can bind to incompatible red blood cells and cause them to be destroyed, resulting in transfusion reactions and other complications.[3]
Serologic methods for blood typing make use of these antibody-antigen reactions. Reagents containing blood group antibodies, called antisera,[1] are added to suspensions of blood cells. If the relevant antigen is present, the antibodies in the reagent will cause the red blood cells to agglutinate (clump together), which can be identified visually.[2] In addition to identifying the ABO antigens, which is termed forward grouping, routine ABO blood typing also includes identification of the ABO antibodies in the person's plasma. This is called reverse grouping,[2] and it is done to confirm the ABO blood type. In reverse grouping, the person's plasma is added to type A1 and type B red blood cells. The plasma should agglutinate the cells that express antigens that the person lacks, while failing to agglutinate cells that express the same antigens as the patient. If this does not occur, further testing is required.[1] Agglutination is scored from 1+ to 4+ based on the strength of the reaction. In ABO typing, a score of 3+ or 4+ indicates a positive reaction, while a score of 1+ or 2+ is inconclusive and requires further investigation.[2]
Blood group antibodies occur in two major forms: Immunoglobulin M (IgM) and Immunoglobulin G (IgG). Antibodies that are predominantly IgM, such as the ABO antibodies, typically cause immediate agglutination of red blood cells at room temperature. Therefore, a person's ABO blood type can be determined by simply adding the red blood cells to the reagent and centrifuging or mixing the sample.[2] RhD typing also typically uses IgM reagents[4]:477 although anti-RhD usually occurs as IgG in vivo.[2] Antibodies that are predominantly IgG, such as those directed towards antigens of the Duffy and Kidd systems,[2] generally do not cause immediate agglutination because the small size of the IgG antibody prevents formation of a lattice structure. Therefore, blood typing using IgG antisera requires incubation at 37 °C (99 °F) and use of the indirect antiglobulin test to demonstrate IgG bound to red blood cells.[2]
To type antigens by the indirect antiglobulin test, antisera against the relevant antigen is added to a suspension of red blood cells, then incubated at 37 °C (99 °F), the ideal temperature for reactivity of IgG antibodies. After incubation, the red blood cells are washed with saline to remove unbound antibodies, and anti-human globulin reagent is added. If the IgG antibodies in the reagent have bound to the antigen on the cell surface, anti-human globulin will bind to those antibodies, causing the red blood cells to agglutinate after centrifugation. If the reaction is negative, "check cells"—reagent cells coated with IgG—are added to ensure that the test is working correctly. If the test result is indeed negative, the check cells should react with the unbound anti-human globulin and demonstrate agglutination.[5]
2. Medical Uses
People who may require a blood transfusion are typed for ABO and RhD to determine which types of blood will be compatible. Blood typing is also routinely performed on pregnant women and on the cord blood from newborn babies, because if the mother's ABO or RhD type is incompatible with the baby's, the baby may be at risk for developing hemolytic disease of the newborn.[6][7] Blood, organ, and hematopoietic stem cell donors have their ABO and RhD types tested before donation to determine which recipients they will be compatible with.[6][7]
2.1. Other Blood Groups
Prior to receiving a blood transfusion, individuals are screened for the presence of antibodies against non-ABO blood group antigens.[7] If a clinically significant antibody is identified, they must be transfused with blood that is negative for the corresponding antigen to prevent a transfusion reaction. This requires the donor units to be typed for the relevant antigen.[2] The recipient is also typed for the antigen to confirm the identity of the antibody, as only individuals who are negative for a blood group antigen should produce antibodies against it.[4]
In Europe, females who require blood transfusions are often typed for the Kell and extended Rh antigens to prevent sensitization to these antigens, which could put them at risk for developing hemolytic disease of the newborn during pregnancy.[8] The American Society of Hematology recommends that people with sickle cell disease have their blood typed for the RhC/c, RhE/e, Kell, Duffy, Kidd, and MNS antigens prior to transfusion,[9] because they often require frequent transfusions and may become sensitized to these antigens if transfused with mismatched blood.[10] Extended red blood cell phenotyping is also recommended for people with beta-thalassemia.[11]
3. Types
3.1. Tube and Slide Methods
Blood typing can be performed using test tubes, microplates, or blood typing slides. The tube method involves mixing a suspension of red blood cells with antisera (or plasma, for reverse grouping) in a test tube. The mixture is centrifuged to separate the cells from the reagent, and then resuspended by gently agitating the tube. If the antigen of interest is present, the red blood cells agglutinate, forming a solid clump in the tube. If it is absent, the red blood cells go back into suspension when mixed.[1][3]The microplate method is similar to the tube method, except rather than using individual test tubes, blood typing is carried out in a plate containing dozens of wells, allowing multiple tests to be performed at the same time. The plate is centrifuged and the agglutination reactions are read.[12]
The slide method involves mixing a drop of blood with a drop of antisera on a slide. The slide is tilted to mix the cells and reagents together and then observed for agglutination, which indicates a positive result. This method is typically used in under-resourced areas or emergency situations; otherwise, alternative methods are preferred.[3]:214[4]:476
3.2. Column Agglutination
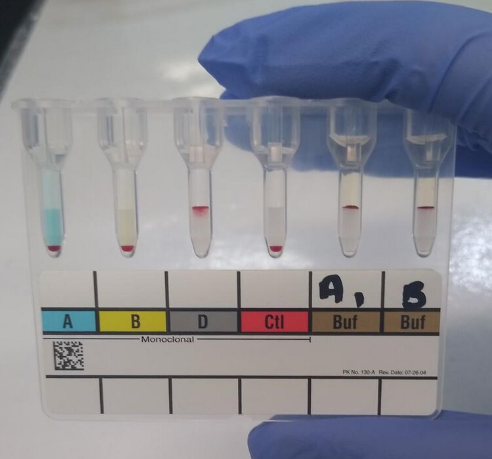
Column agglutination techniques for blood typing (sometimes called a "gel test") involve placing suspensions of red blood cells onto cards containing columns of dextran-polyacrylamide gel. The columns may contain pre-dispensed blood typing reagents, or plasma may be added for reverse grouping. The gel cards are then centrifuged. Red blood cell agglutinates are too large to migrate through the gel and become trapped at the top of the column, while unagglutinated cells collect on the bottom. Therefore, a line of red blood cells at the top of the column indicates a positive result. The strength of positive reactions is scored from 1+ to 4+ depending on how far the cells have travelled through the gel. The gel test has advantages over manual methods in that it eliminates the variability associated with manually re-suspending the cells and that the cards can be kept as a record of the test.[2] The column agglutination method is used by some automated analyzers to perform blood typing automatically.[1] These analyzers pipette red blood cells and plasma onto gel cards, centrifuge them, and scan and read the agglutination reactions to determine the blood type.[2]
3.3. Solid-Phase Assay
Solid-phase assays (sometimes called the "antigen capture" method) use reagent antigens or antibodies affixed to a surface (usually a microplate).[3] Microplate wells coated with anti-A, -B and -D reagents are used for forward grouping, while reagent red cells are added to empty wells for reverse grouping. The test sample is added and the microplate is centrifuged. In a positive reaction, the red blood cells adhere to the surface of the well.[1][4] Some automated analyzers use solid phase assays for blood typing.[2]
3.4. Genotyping
Genetic testing can be used to determine a person's blood type in certain situations where serologic testing is insufficient. For example, if a person has been transfused with large volumes of donor blood, the results of serologic testing will reflect the antigens on the donor cells and not the person's actual blood type.[8] Individuals who produce red blood cell autoantibodies[12] or who are treated with certain drugs may show spurious agglutination reactions in serologic testing, so genotyping may be necessary to determine the blood type accurately.[2] Genetic testing is required for typing red blood cell antigens for which no commercial antisera are available.[8]
The American Association of Blood Banks recommends RhD antigen genotyping for women with serologic weak D phenotypes who have the potential to bear children. This is because some people with weak D phenotypes can produce antibodies against the RhD antigen, which can cause hemolytic disease of the newborn, while others cannot. Genotyping can identify the specific type of weak D antigen, which determines the potential for the person to produce antibodies, thus avoiding unnecessary treatment with Rho(D) immune globulin.[13][14] Genotyping is preferred to serologic testing for people with sickle cell disease, because it is more accurate for certain antigens and can identify antigens that cannot be detected by serologic methods.[10]
Genotyping is also used in prenatal testing for hemolytic disease of the newborn. When a pregnant woman has a blood group antibody associated with HDN, the fetus can be typed for the relevant antigen to determine if it is at risk of developing the disease. Because it is impractical to draw blood from the fetus, the blood type is determined using an amniocentesis sample or cell-free fetal DNA isolated from the mother's blood.[12][13] The father may also be genotyped to predict the risk of hemolytic disease of the newborn, because if the father is homozygous for the relevant antigen (meaning having two copies of the gene) the baby will be positive for the antigen and thus at risk of developing the disease. If the father is heterozygous (having only one copy), the baby only has a 50% chance of being positive for the antigen.[8]
4. Limitations
4.1. ABO Discrepancies
In ABO typing, the results of the forward and reverse grouping should always correspond with each other. An unexpected difference between the two results is termed an ABO discrepancy, and must be resolved before the person's blood type is reported.[2]
Forward grouping
Weak reactions in the forward grouping may occur in people who belong to certain ABO subgroups—variant blood types characterized by decreased expression of the A or B antigens or changes in their structure. Weakened expression of ABO antigens may also occur in leukemia and Hodgkin's lymphoma. Weak reactions in forward grouping can be strengthened by incubating the blood and reagent mixture at room temperature or 4 °C (39 °F), or by using certain enzymes to enhance the antigen-antibody reactions.[2]
Occasionally, two populations of red blood cells are apparent after reaction with the blood typing antisera. Some of the red blood cells are agglutinated, while others are not, making it difficult to interpret the result. This is called a mixed field reaction, and it can occur if someone has recently received a blood transfusion with a different blood type (as in a type A patient receiving type O blood), if they have received a bone marrow or stem cell transplant from someone with a different blood type, or in patients with certain ABO subgroups, such as A3. Investigation of the person's medical history can clarify the cause of the mixed field reaction.[2][15]
People with cold agglutinin disease produce antibodies against their own red blood cells that cause them to spontaneously agglutinate at room temperature, leading to false positive reactions in forward grouping. Cold agglutinins can usually be deactivated by warming the sample to 37 °C (99 °F) and washing the red blood cells with saline. If this is not effective, dithiothreitol can be used to destroy the antibodies.[2]
Cord blood samples may be contaminated with Wharton's jelly, a viscous substance that can cause red blood cells to stick together, mimicking agglutination. Wharton's jelly can be removed by thoroughly washing the red blood cells.[2]
In a rare phenomenon known as "acquired B antigen", a patient whose true blood type is A may show a weak positive result for B in the forward grouping. This condition, which is associated with gastrointestinal diseases such as colon cancer[2] and intestinal obstruction,[15] results from conversion of the A antigen to a structure mimicking the B antigen by bacterial enzymes.[4] Unlike the true B antigen, acquired B antigen does not react with reagents within a certain pH range.[2]
Reverse grouping
Infants under 3 to 6 months of age exhibit missing or weak reactions in reverse grouping because they produce very low levels of ABO antibodies.[2] Therefore, reverse grouping is generally not performed for this age group.[4] Elderly people may also exhibit decreased antibody production, as may people with hypogammaglobulinemia. Weak reactions can be strengthened by allowing the plasma and red cells to incubate at room temperature for 15 to 30 minutes, and if this is not effective, they can be incubated at 4 °C (39 °F).[2]
Approximately 20% of individuals with the blood type A or AB belong to a subgroup of A, termed A2, while the more common subgroup, encompassing approximately 80% of individuals, is termed A1. Because of small differences in the structure of the A1 and A2 antigens, some individuals in the A2 subgroup can produce an antibody against A1. Therefore, these individuals will type as A or AB in the forward grouping, but will exhibit an unexpected positive reaction with the type A1 red cells in the reverse grouping. The discrepancy can be resolved by testing the person's red blood cells with an anti-A1 reagent, which will give a negative result if the patient belongs to the A2 subgroup. Anti-A1 antibodies are considered clinically insignificant unless they react at 37 °C (99 °F). Other subgroups of A exist, as well as subgroups of B, but they are rarely encountered.[2]
If high levels of protein are present in a person's plasma, a phenomenon known as rouleaux may occur when their plasma is added to the reagent cells. Rouleaux causes red blood cells to stack together, which can mimic agglutination, causing a false positive result in the reverse grouping. This can be avoided by removing the plasma, replacing it with saline, and re-centrifuging the tube. Rouleaux will disappear once the plasma is replaced with saline, but true agglutination will persist.[2]:139
Antibodies to blood group antigens other than A and B may react with the reagent cells used in reverse grouping. If a cold-reacting autoantibody is present, the false positive result can be resolved by warming the sample to 37 °C (99 °F). If the result is caused by an alloantibody, an antibody screen can be performed to identify the antibody,[1] and the reverse grouping can be performed using samples that lack the relevant antigen.[4]
4.2. Weak D Phenotype
Approximately 0.2 to 1% of people have a "weak D" phenotype,[16] meaning that they are positive for the RhD antigen, but exhibit weak or negative reactions with some anti-RhD reagents due to decreased antigen expression or atypical variants of antigen structure. If routine serologic testing for RhD results in a score of 2+ or less, the antiglobulin test can be used to demonstrate the presence of RhD.[2] Weak D testing is also performed on blood donors who initially type as RhD negative.[13] Historically, blood donors with weak D were treated as Rh positive and patients with weak D were treated as Rh negative in order to avoid potential exposure to incompatible blood. Genotyping is increasingly used to determine the molecular basis of weak D phenotypes, as this determines whether or not individuals with weak D can produce antibodies against RhD or sensitize others to the RhD antigen.[16]
4.3. Red Cell Antibody Sensitization
The indirect antiglobulin test, which is used for weak D testing and typing of some red blood cell antigens, detects IgG bound to red blood cells. If IgG is bound to red blood cells in vivo, as may occur in autoimmune hemolytic anemia, hemolytic disease of the newborn and transfusion reactions,[4] the indirect antiglobulin test will always give a positive result, regardless of the presence of the relevant antigen.[4]A direct antiglobulin test can be performed to demonstrate that the positive reaction is due to sensitization of red cells.[17]
5. History

In 1901, Karl Landsteiner published the results of an experiment in which he mixed the serum and red blood cells of five different human donors. He observed that a person's serum never agglutinated their own red blood cells, but it could agglutinate others', and based on the agglutination reactions the red cells could be sorted into three groups: group A, group B, and group C. Group C, which consisted of red blood cells that did not react with any person's plasma, would later be known as group O.[18] A fourth group, now known as AB, was described by Landsteiner's colleagues in 1902.[18] This experiment was the first example of blood typing.[2]
In 1945, Robin Coombs, A.E. Mourant and R.R. Race published a description of the antiglobulin test (also known as the Coombs test). Previous research on blood group antibodies had documented the presence of so-called "blocking" or "incomplete" antibodies: antibodies that occupied antigen sites, preventing other antibodies from binding, but did not cause red blood cells to agglutinate. Coombs and his colleagues devised a method to easily demonstrate the presence of these antibodies. They injected human immunoglobulins into rabbits, which caused them to produce an anti-human globulin antibody. The anti-human globulin could bind to antibodies already attached to red blood cells and cause them to agglutinate. The invention of the antiglobulin test led to the discovery of many more blood group antigens. By the early 1950s, companies had begun producing commercial antisera for special antigen testing.[18]
The content is sourced from: https://handwiki.org/wiki/Medicine:Blood_typing
References
- Mary Louise Turgeon (10 February 2015). Linne & Ringsrud's Clinical Laboratory Science - E-Book: The Basics and Routine Techniques. Elsevier Health Sciences. ISBN 978-0-323-37061-5. https://books.google.com/books?id=D1umBgAAQBAJ.
- Denise M Harmening (30 November 2018). Modern Blood Banking & Transfusion Practices. F.A. Davis. ISBN 978-0-8036-9462-0. https://books.google.com/books?id=vxyDDwAAQBAJ.
- Jeffrey McCullough (27 September 2016). Transfusion Medicine. Wiley. ISBN 978-1-119-23652-8. https://books.google.com/books?id=5YAsDQAAQBAJ.
- Bain, BJ; Bates, I; Laffan, MA (11 August 2016). Dacie and Lewis Practical Haematology E-Book. Elsevier Health Sciences. ISBN 978-0-7020-6925-3. https://books.google.com/books?id=rEPUDAAAQBAJ.
- Richard A. McPherson; Matthew R. Pincus (6 September 2011). Henry's Clinical Diagnosis and Management by Laboratory Methods. Elsevier Health Sciences. ISBN 1-4557-2684-2. https://books.google.com/books?id=-97J-8Zh2hkC&pg=PA714.
- American Association for Clinical Chemistry (15 November 2019). "Blood Typing". Lab Tests Online. https://labtestsonline.org/tests/blood-typing. Retrieved 27 January 2020.
- Gonsorcik, V.K. (7 August 2018). "ABO Grouping: Overview, Clinical Indications/Applications, Test Performance". Medscape. https://emedicine.medscape.com/article/1731198-overview#a2. Retrieved 2 March 2020.
- Westhoff, Connie M. (2019). "Blood group genotyping". Blood 133 (17): 1814–1820. doi:10.1182/blood-2018-11-833954. ISSN 0006-4971. PMID 30808639. https://dx.doi.org/10.1182%2Fblood-2018-11-833954
- Callum, JL; Pinkerton, PH; Lima, A (2016) (PDF). Bloody Easy 4: Blood transfusions, blood alternatives and transfusion reactions. Ontario Regional Blood Coordinating Network. ISBN 978-0-9869176-2-2. https://transfusionontario.org/en/download/bloody-easy-4-blood-transfusions-blood-alternatives-and-transfusion-reactions-a-guide-to-transfusion-medicine-fourth-edition/.
- Chou, Stella T.; Alsawas, Mouaz; Fasano, Ross M.; Field, Joshua J.; Hendrickson, Jeanne E.; Howard, Jo; Kameka, Michelle; Kwiatkowski, Janet L. et al. (2020). "American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support". Blood Advances 4 (2): 327–355. doi:10.1182/bloodadvances.2019001143. ISSN 2473-9529. PMID 31985807. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6988392
- Compernolle, Veerle; Chou, Stella T.; Tanael, Susano; Savage, William; Howard, Jo; Josephson, Cassandra D.; Odame, Isaac; Hogan, Christopher et al. (2018). "Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline". Transfusion 58 (6): 1555–1566. doi:10.1111/trf.14611. ISSN 00411132. PMID 29697146. https://dx.doi.org/10.1111%2Ftrf.14611
- A. Victor Hoffbrand; Douglas R. Higgs; David M. Keeling; Atul B. Mehta (28 October 2015). Postgraduate Haematology. Wiley. ISBN 978-1-118-85447-1. https://books.google.com/books?id=Rq7YCgAAQBAJ.
- Sandler, S. Gerald; Flegel, Willy A.; Westhoff, Connie M.; Denomme, Gregory A.; Delaney, Meghan; Keller, Margaret A.; Johnson, Susan T.; Katz, Louis et al. (2015). "It's time to phase in RHD genotyping for patients with a serologic weak D phenotype". Transfusion 55 (3): 680–689. doi:10.1111/trf.12941. ISSN 00411132. PMID 25438646. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4357540
- American Association of Blood Banks. "Joint Statement on Phasing-In RHD Genotyping for Pregnant Women and Other Females of Childbearing Potential with a Serologic Weak D Phenotype". American Association of Blood Banks – Statements. http://www.aabb.org/advocacy/statements/Pages/statement150722.aspx.
- Harvey G. Klein; David J. Anstee (3 February 2014). Mollison's Blood Transfusion in Clinical Medicine. John Wiley & Sons. ISBN 978-1-4051-9940-7. https://books.google.com/books?id=daQTAgAAQBAJ&pg=PA325.
- Sandler, S. Gerald; Chen, Leonard N.; Flegel, Willy A. (2017). "Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype". British Journal of Haematology 179 (1): 10–19. doi:10.1111/bjh.14757. ISSN 00071048. PMID 28508413. https://dx.doi.org/10.1111%2Fbjh.14757
- Sally V. Rudmann (18 February 2005). Textbook of Blood Banking and Transfusion Medicine. Elsevier Health Sciences. ISBN 0-7216-0384-X. https://books.google.com/books?id=dXdISwJQJFIC&pg=PA289.
- Marion E. Reid; Ian Shine (2012). The Discovery and Significance of the Blood Groups. SBB Books. ISBN 978-1-59572-422-9. https://books.google.com/books?id=n2aEtgAACAAJ.
