Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bioactive amygdalin, found in high concentrations in bitter almonds, has been recognized as a symbol of the cyanogenic glycoside chemical organic substance, which was initially developed as a pharmaceutical for treating cancer after being hydrolyzed to hydrogen cyanide (HCN).
- amygdalin
- antioxidant
- anticancer
- microbiota intervention
1. Introduction
Amygdalin is a primary active pharmaceutical ingredient in almonds and is also commonly found in the seeds of Rosaceae species [1][2]. It is a naturally occurring substance that can be discovered in the seeds of many different plants [3]. The chemical formula for cyanogenic glycoside is C20H27O11 (Figure 1A), and it has a molecular mass of 457.42 g mol−1. Benzaldehyde, hydrocyanic acid, and two glucose molecules (D-mandelopnitrile-β-D-glucoside-6-β-glucoside), also known as Laeteil, make up amygdalin, a compound found in almond, apricot, and apple seeds [4]. Some of its qualities include anti-inflammatory, antibacterial, antioxidant, and immunomodulatory effects [3][5]. Hydrogen cyanide (HCN), a byproduct of amygdalin’s enzymatic hydrolysis, is dangerous, while amygdalin itself is harmless. Amygdalin’s positive benefits have been studied for decades, and the results have been consistently positive across a wide range of medical conditions, including but not limited to leprosy, colorectal cancer, asthma, bronchitis, and others [6][7]. Benzaldehyde, which is present in its molecule, makes it an analgesic [8]. However, the efficiency of its anticancer activity is still up for debate and requires additional research. This ability may be related to hydrocyanic acid emission during enzymatic hydrolysis [9]. Questions concerning amygdalin’s effectiveness as an anticancer treatment have been raised due to its toxicity to healthy cells and its limited pharmacokinetic properties. Inhibiting the growth of cancer cells by eliminating carcinogenic substances is assumed to be the primary anticancer activity. It is known as apoptosis [10], preventing the food supply to cancer cells, which reduces the prevalence of many types of cancer [11].
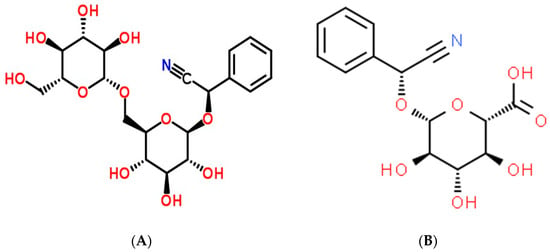
Figure 1. Amygdalin (A) and laetrile (B) chemical structures.
2. History and Structural Characteristics of Amygdalin as an Anticancer Therapy
Robiquet and Boutron-Charlard, two French chemists, discovered amygdalin (which was named “emulsin”) from bitter almonds in 1837 [12]. In 1845, it was tested as a potential cancer treatment in Russia, but it was quickly abandoned due to its high toxicity and poor efficacy. Germany rejected this cancer treatment in 1892. U.S. records show it was first used to cure cancer in the 1920s, but a subsequent clinical assessment by Dr. Ernst Krebs proved its danger to people. In the 1950s, a patent was issued for laetrile, an intravenous version of amygdalin that was said to be safe for human consumption. The National Cancer Institute (NCI) studied the quality of amygdalin products manufactured by Cyto Pharma of Mexico and found that both the oral and injectable forms did not exceed US pharmaceutical safety guidelines [7][13]. Therefore, it was rejected by the Food and Drug Administration (FDA). However, amygdalin had become one of the most popular anticancer approaches in the 1970s when it was reported that 70,000 American cancer patients were using it as a complementary and alternative therapy [14]. Twenty-three US states legalized its usage in 1980 for those with terminal cancer. Two FDA-approved clinical trials supported by the NCI in the 1980s showed that laetrile did not work.
The importation of amygdalin was outlawed in the United States and Mexico in 1987. However, laetrile is still produced and used as an anticancer treatment, particularly in Mexico [15]. Due to its cyanide toxicity, amygdalin is restricted to medical use in the United Kingdom [16][17]. Some studies equated amygdalin with vitamin B17, whereas others equated laetrile, a semi-synthetic injectable version of amygdalin, with vitamin B17 (Figure 1B) [18]. Commonly, people will use amygdalin, laetrile, or vitamin B17 interchangeably. This study will therefore stick to the research term employed in the primary sources. The amygdalin dose for in vivo research is provided in milligrams per kilogram of body weight (mg kg−1), while the in vitro dose is expressed in molar concentrations. Future amygdalin applications were considered in the context of amygdalin and other cyanogenetic glucosides as anticancer medications and potential anticancer agents [19].
3. In Vivo and In Vitro Antioxidant Potential of Amygdalin
Fruit stones have a high protein concentration in the kernel that can be used for other purposes. In addition to carbs and lipids, the embryo must store this substance for use throughout the earliest stages of development. Some fruit seed proteins are valued for their physicochemical features in addition to their nutritional benefits [20], benefits in complementary and alternative medicine [21], and also for serving as a rich source of bioactive peptides [22]. However, cyanogenic glycosides such as Amygdalin make it challenging to extract helpful protein from these renewable sources. Hydrolysis enzymes are generated when the plant cell structure is damaged, although amygdalin is non-toxic. By breaking down amygdalin, researchers obtain benzaldehyde, which has a bitter taste, and cyanide, which is toxic [23]. Numerous plants contain amygdalin, including almonds, pecans, cereals, and legumes. However, meals and beverages derived from these sources often have low levels of cyanide [24]. The legal limit of cyanogenic glycosides in foods is set by government legislation. For example, the European Food Safety Authority has set limits of cyanide levels of 35 mg kg−1 in alcoholic beverages, 5 mg kg−1 in canned stone fruits, and 50 mg kg−1 in nougat, marzipan, or their replacements or comparable goods [25].
Uniquely, apricot seeds include antioxidants, an Angiotensin I converting enzyme (ACE) inhibitor, and hypocholesterolemic peptides [26]. The antioxidant, antimicrobial, anti-inflammatory, and immune-regulating properties of amygdalin are in addition to its effectiveness against tumors [27]. The metabolizing enzymes rhodanese (RHD) and betaglucosidase (BGD) control their anticancer action in vivo. After treating Balb/c nude mice with amygdalin, Alwan and Afshari [27] found that the metabolic enzymes RHD and BGD played a crucial role in boosting the antigrowth of PC3 cancer cell lines. This provided insight into a potential mechanism of action for amygdalin, Figure 2. In contrast to high-dose amygdalin, which negatively affected the oxidative balance of male mice’s hepatic and testicular tissues, low- and medium-dose amygdalin had no such effect. Thus, amygdalin, in low doses, may restore oxidative equilibrium in mice [28]. Moreover, amygdalin exhibits significant antioxidant activity in the liver tissues and suppresses tunicamycin-induced endoplasmic reticulum stress in mice [29]. Amygdalin was isolated from Prunus dulcis, and its antioxidant and cytotoxic characteristics were investigated in vitro by Sushma et al. [30]. Multiple antioxidant experiments indicated that the P. dulcis amygdalin extract possessed strong antioxidant properties. The cytotoxic activity of amygdalin against HeLa cancer cells was found to be relatively high, and it possessed promising biological features overall. As its ethanolic extract of C. horizontalis branches contains natural vitamin E (0.76 mg 100 g−1 extract) and amygdalin, it was suggested by Sokkar et al. [31] that it may be a source of a natural antioxidant with hepatoprotective and hypolipidemic effects (0.11 mg 100 g−1 extract). Complete M.S. medium containing 2 mg L−1 BAP or 6 mg L−1 of naphthalene acetic acid stimulated vitamin E production in vitro cells, and a complete M.S. medium containing 4 mg L−1 kin or 2 mg L−1 2,4 D stimulated amygdalin production.
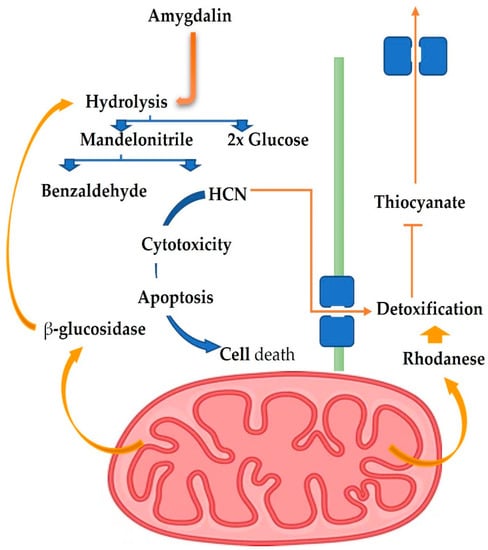
Figure 2. Controlling AMD in cancer and normal cells: the function of RHD and BGD [27].
This entry is adapted from the peer-reviewed paper 10.3390/biom12101514
References
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of The Gut Microbiota on Amygdalin and Its Use as an Anti-Cancer Therapy: Substantial Review on the Key Components Involved in Altering Dose Efficacy and Toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132.
- Toomey, V.M.; Nickum, E.A.; Flurer, C.L. Cyanide and Amygdalin as Indicators of the Presence of Bitter Almonds in Imported Raw Almonds. J. Forensic Sci. 2012, 57, 1313–1317.
- Orlikova, B.; Legrand, N.; Panning, J.; Dicato, M.; Diederich, M. Anti-Inflammatory and Anticancer Drugs from Nature. In Advances in Nutrition and Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–143.
- Qadir, M.; Fatima, K. Review on Pharmacological Activity of Amygdalin. Arch. Cancer Res. 2017, 5, 160.
- Barakat, H. Amygdalin as A Plant-Based Bioactive Constituent: An Updated Substantial Review on Intervention with Gut Microbiota, Anticancer Therapy, Bioavailability, and Microencapsulation. In Proceedings of the 1st International Electronic Conference on Nutrients—Nutritional and Microbiota Effects on Chronic Disease session Potential nutraceutical effects of nutrients, phytochemicals, and microbiota in chronic metabolic disorders, online, 2–15 November 2020.
- Li, X.-B.; Liu, C.-H.; Zhang, R.; Huang, X.-T.; Li, Y.-Y.; Han, L.; Xu, M.-L.; Mi, S.-Q.; Wang, N.-S. Determination and Pharmacokinetics of Amygdalin in Rats By LC–MS-MS. J. Chromatogr. Sci. 2014, 52, 476–481.
- Song, Z.; Xu, X. Advanced Research on Anti-tumor Effects of Amygdalin. J. Cancer Res. 2014, 10, 3.
- Chang, H.-K.; Shin, M.-S.; Yang, H.-Y.; Lee, J.-W.; Kim, Y.-S.; Lee, M.-H.; Kim, J.; Kim, K.-H.; Kim, C.-J. Amygdalin Induces Apoptosis Through Regulation of Bax and Bcl-2 Expressions in Human Du145 and Lncap Prostate Cancer Cells. Biol. Pharm. Bull. 2006, 29, 1597–1602.
- Zhou, C.; Qian, L.; Ma, H.; Yu, X.; Zhang, Y.; Qu, W.; Zhang, X.; Xia, W. Enhancement of Amygdalin Activated with Β-D-Glucosidase on HEPG2 Cells Proliferation and Apoptosis. Carbohydr. Polym. 2012, 90, 516–523.
- Park, H.-J.; Yoon, S.-H.; Han, L.-S.; Zheng, L.-T.; Jung, K.-H.; Uhm, Y.-K.; Lee, J.-H.; Jeong, J.-S.; Joo, W.-S.; Yim, S.-V. Amygdalin Inhibits Genes Related to Cell Cycle in Snu-C4 Human Colon Cancer Cells. World J. Gastroenterol. 2005, 11, 5156.
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin Induces Apoptosis in Human Cervical Cancer Cell Line Hela Cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51.
- Dorr, R.T.; Paxinos, J. The Current Status of Laetrile. Ann. Intern. Med. 1978, 89, 389–397.
- Davignon, J.P.; Trissel, L.A.; Kleinman, L.M. Pharmaceutical Assessment of Amygdalin (Laetrile) Products. Cancer Treat. Rep. 1978, 62, 99–104.
- Moertel, C.G.; Fleming, T.R.; Rubin, J.; Kvols, L.K.; Sarna, G.; Koch, R.; Currie, V.E.; Young, C.W.; Jones, S.E.; Davignon, J.P. A Clinical Trial of Amygdalin (Laetrile) in The Treatment of Human Cancer. N. Engl. J. Med. 1982, 306, 201–206.
- Society, A.C. Actualización de American Cancer Society 1991.Questionable Cancer Practices in Tijuana and Other Mexican Border Clinics. Cancer J. Clin. 1991, 41, 310–319.
- Milazzo, S.; Lejeune, S.; Ernst, E. Laetrile for Cancer: A Systematic Review of the Clinical Evidence. Support. Care Cancer 2007, 15, 583–595.
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin Content of Seeds, Kernels and Food Products Commercially-Available in The Uk. Food Chem. 2014, 152, 133–139.
- Sireesha, D.; Reddy, B.S.; Reginald, B.A.; Samatha, M.; Kamal, F. Effect of Amygdalin on Oral Cancer Cell Line: An in Vitro Study. J. Oral Maxillofac. Pathol. 2019, 23, 104.
- Shi, J.; Chen, Q.; Xu, M.; Xia, Q.; Zheng, T.; Teng, J.; Li, M.; Fan, L. Recent Updates and Future Perspectives about Amygdalin as A Potential Anticancer Agent: A Review. Cancer Med. 2019, 8, 3004–3011.
- Mirhosseini, H.; Amid, B.T. Effect of Different Drying Techniques on Flowability Characteristics and Chemical Properties of Natural Carbohydrate-Protein Gum from Durian Fruit Seed. Chem. Cent. J. 2013, 7, 44.
- Binita, K.; Kumar, S.; Sharma, V.K.; Sharma, V.; Yadav, S. Proteomic Identification of Syzygium cumini Seed Extracts by MALDI-TOF/MS. Appl. Biochem. Biotechnol. 2014, 172, 2091–2105.
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and Angiotensin I Converting Enzyme (Ace) Inhibitory Activities of Date Seed Protein Hydrolysates Prepared Using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137.
- Stosic, D.; Gorunovic, M.; Popovic, B. Preliminary Toxicological Study of The Kernel and The Oil of Some Prunus Species. Plantes Med. Et. Phytother. 1987, 21, 8–13.
- Tunçel, G.; Nout, M.J.R.; Brimer, L. Degradation of Cyanogenic Glycosides of Bitter Apricot Seeds (Prunus Armeniaca) by Endogenous and Added Enzymes as Affected by Heat Treatments and Particle Size. Food Chem. 1998, 63, 65–69.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; et al. Evaluation of the Health Risks Related to the Presence of Cyanogenic Glycosides in Foods other than Raw Apricot Kernels. EFSA J. 2019, 17, e05662.
- Garcia, M.C.; Gonzalez-Garcia, E.; Vasquez-Villanueva, R.; Marina, M.L. Apricot and other Seed Stones: Amygdalin Content and the Potential to Obtain Antioxidant, Angiotensin I Converting Enzyme Inhibitor and Hypocholesterolemic Peptides. Food Funct. 2016, 7, 4693–4701.
- Alwan, A.M.; Afshari, J.T. In Vivo Growth Inhibition of Human Caucasian Prostate Adenocarcinoma in Nude Mice Induced by Amygdalin with Metabolic Enzyme Combinations. Biomed. Res. Int. 2022, 2022, 4767621.
- Albogami, S.; Hassan, A.; Ahmed, N.; Alnefaie, A.; Alattas, A.; Alquthami, L.; Alharbi, A. Evaluation of the Effective Dose of Amygdalin for the Improvement of Antioxidant Gene Expression and Suppression of Oxidative Damage in Mice. PeerJ 2020, 8, e9232.
- Moslehi, A.; Komeili-movahed, T.; Moslehi, M. Antioxidant Effects of Amygdalin on Tunicamycin-Induced Endoplasmic Reticulum Stress in The Mice Liver: Cross Talk Between Endoplasmic Reticulum Stress and Oxidative Stress. J. Rep. Pharm. Sci. 2019, 8, 298.
- Sushma, P.; Jacob, B.; Narendhirakannan, R.T. Evaluation of Antioxidant And Cytotoxicity Properties Of Amygdalin Extracted From Prunus Dulcis. Kongunadu Res. J. 2019, 6, 8–12.
- Sokkar, N.; El-Gindi, O.; Sayed, S.; Mohamed, S.; Ali, Z.; Alfishawy, I. Antioxidant, Anticancer and Hepatoprotective Activities of Cotoneaster Horizontalis Decne Extract as well as A-Tocopherol and Amygdalin Production from in Vitro Culture. Acta Physiol. Plant. 2013, 35, 2421–2428.
This entry is offline, you can click here to edit this entry!
