Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pedometers and accelerometers have become commonplace for the assessment of physical behaviors (e.g., physical activity and sedentary behavior) in multiple sclerosis (MS) research. Current common applications include the measurement of steps taken and the classification of physical activity intensity, as well as sedentary behavior, using cut-points methods.
- physical activity
- sedentary behavior
- wearable technology
- multiple sclerosis
1. Introduction
Wearable motion sensors, namely pedometers and accelerometers, have been instrumental in improving the assessment of physical behaviors in research studies, especially physical activity, sedentary behavior, and sleep. Of note, data collected with these devices have allowed for a better understanding of the associations between physical activity and health outcomes in different populations [1]. Within the field of multiple sclerosis (MS), the use of wearable motion sensors for measuring physical activity behavior has advanced significantly over the last two decades [2].
The growing use of wearable motion sensors in MS is a result of physical activity being currently recognized as an essential therapeutic component [3], as it has been consistently associated with beneficial effects for MS symptoms and function [4][5]. Physical activity is traditionally defined as any bodily movement produced by the contraction of the skeletal muscles that results in increased energy expenditure [6], whereas sedentary behavior is defined as any waking activity performed in a seated, reclined, or lying position with an energy expenditure ≤1.5 MET [7]. To date, the majority of studies on motion sensors in MS have focused on examining the validity and/or reliability of pedometers and/or accelerometers in assessing steps taken and physical activity-related metrics, such as energy expenditure or time spent in sedentary behavior and different physical activity intensities [8]. These applications have allowed for significant progress in understanding how device-measured physical activities and sedentary behaviors are related with health in persons with MS. However, there are major opportunities and possibilities for further applying wearable motion sensors and exploring the resulting data for identifying signatures representing free-living physical function and mobility status in MS.
2. Applications of Motion Sensors in MS
2.1. Physical Activity Assessment
The majority of the current wearable motion sensors are of small size, can be placed on different body parts, and use piezoelectric, piezoresistive, or capacitive sensing mechanisms [1]. The most commonly used wearable motion sensors in MS have been pedometers and accelerometer-based activity monitors [8]. These devices allow for the collection of different measures related to physical activity, such as number of steps, step-rate, kcals/min, raw acceleration (g-force), and activity counts. Studies have tested the validity and reliability of these devices in MS and have developed physical activity prediction methods specifically for this group of people.
Overall, most pedometers have been demonstrated to be accurate in recording steps during regular and fast walking speeds in MS [9][10]. Regarding slow walking speeds (e.g., ≤54 m/min), piezoelectric- or accelerometer-based pedometers are more accurate than spring-levered pedometers [11][12]. Of note, accuracy of pedometers in persons with MS depends on the disability level, with a higher measurement error occurring for those with severe MS disability, as demonstrated by Sandroff et al. [13], where the accuracy of the ActiGraph GT3X activity monitor decreased from 95.5% to 87.3% relative to manually counted steps. Conversely, the StepWatch, which is a gold-standard device for capturing steps in clinical populations, only demonstrated a very small decrement in accuracy during slow walking speeds in persons with severe MS disability (99% to 95.7%, relative to manually counted steps) [13]. Such results denote that the StepWatch may better differentiate the mobility status in free-living situations than the ActiGraph GT3X if step is the metric of interest. The high accuracy of the StepWatch establishes it as a reference method that can be used to validate other pedometers under free-living conditions [8], since there is a lack of such devices that have truly undergone ecological validity testing.
Regarding accelerometry, researchers have traditionally applied cut-points to classify physical activity intensity in MS [8]. This method involves the translation of accelerometer output into meaningful metrics of physical activity, a process termed calibration, wherein researchers establish the relationship between accelerometer output (i.e., counts/minute) and energy expenditure (i.e., metabolic equivalents (METs)) [1][14]. Within the field of MS, several disturbances in physical function, including altered gait parameters (e.g., walking velocity, cadence, step length, and stride length), result in a higher oxygen cost (O2 cost) during ambulation, compared with the general population [15][16], and this manifests as higher MET values per a given number of activity counts per minute. To address this issue, two studies [17][18] have established accelerometer cut-points for fully ambulatory persons with MS, and another study [15] has derived cut-points for persons with MS according to disability status. Accounting for the disability status is important because it identifies differences in the relationship of accelerometer output with oxygen cost for the activities, and further because as disability increases, locomotion speed tends to decrease. As demonstrated by Sandroff et al. [15], for individuals with mild disability, slow, comfortable, and fast walking speeds were 2.22, 2.84, and 3.21 mph, whereas for individuals with severe disability, the speeds for the same categories were 0.97, 1.40, and 1.74 mph, respectively. Thus, it becomes clear that one should not apply the same cut-points for individuals with different disability statuses, as the functional capacity is highly different, meaning that the cut-points for individuals with mild MS disability will be too high for individuals with severe MS disability. This indicates that researchers should not apply cut-points developed for the general population among individuals with MS. The MS-specific cut-points have been important for improving the understanding of associations between physical activity and different health outcomes in MS [19], both in observational and experimental studies [20][21][22][23].
2.2. Sedentary Behavior Assessment and Interruption
Sedentary behavior assessment in MS has gained attention because of its negative associations with health outcomes [24]. Accelerometers allow for capturing the total time spent on sedentary behavior, and further, the number of breaks in such behavior, as well as the durations of these breaks. Some current motion sensors and prediction algorithms allow for the detection of postural transitions (e.g., sit-to-stand or vice versa), and this may provide an indication of lower body function under free-living conditions [25].
Studies using accelerometers suggest that persons with MS with mobility disability spend 65% (8.9 h/day) of their daily time in sedentary behavior, compared with 60% (8.4 h/day) for those without mobility disability [26]. The number of bouts in sedentary behavior lasting more than 30 min was slightly higher in persons with MS with mobility disability compared with those without mobility disability (5.1 vs. 4.3 bouts, p = 0.02) [26]. Accelerometer data further indicate that sedentary behavior is higher in older adults with MS compared with middle-aged and young adults with MS [27], and that longer durations of sedentary bouts partially correlate with lower physical and cognitive functions in older adults with MS [28]. These studies have been instrumental for establishing the deleterious associations of sedentary behavior with mobility disability and health outcomes in MS. However, the studies lack the power for inferring causal relationships. One next step in studies using accelerometers to examine sedentary behavior in MS is to apply such devices longitudinally for long periods of time, comprehending periods of relapses and/or disease worsening, allowing for a better understanding of how mobility disability, as well as physical and cognitive functions, relate to changes in sedentary behavior over time.
Besides only monitoring sedentary behavior, wearable motion sensors may be applied for promoting interruptions of prolonged sitting in MS. Some devices, such as the activPAL, already present buzzing features for alerting individuals about interrupting sitting at given intervals (e.g., hourly or every two hours). These prompting signals may help with examining the potential health benefits of interrupting sedentary time and replacing it with light or moderate physical activity [29][30]. Therefore, in addition to only monitoring physical behaviors, motion sensors may be used to reduce sedentary behavior and increase physical activity in MS.
3. Opportunities for Using Motion Sensors in MS
3.1. Biomarkers of Disease Severity and Progression
Overall, the field has witnessed substantial progress in using wearable motion sensors for assessing physical activity and sedentary behavior in MS. As the technology for the devices and data processing methods are evolving, new opportunities for assessing physical behaviors and clinical outcomes have emerged. Motion sensor data have the potential for tracking disease progression and severity based on the ecologically valid assessment of physical function and mobility [5]. Such an approach would bring additional information to existing laboratory-based tests.
There is evidence that free-living accelerometer data may be related to performances on different mobility tests. Studies have indicated that ActiGraph 7164 activity counts were strongly correlated with walking speed (r = 0.82) [31] and mobility measures, such as the 6 min walk test (ρ = 0.78), and the Timed Up and Go test (ρ = −0.68) [32]. Similarly, pedometer output (step counts) has been correlated with measures of walking performance, mobility, and disability over a 7-day period (EDSS (ρ = −0.90), Multiple Sclerosis Walking Scale-12 (MSWS-12; ρ = −0.83), Timed 25-Foot Walk (ρ = −0.64), Timed Up and Go test (ρ = −0.51), and 6 min walk test (ρ = 0.67)) [33].
Those results provided a proof-of-concept that free-living accelerometer data may be related to physical function, and recent evidence suggests that unsupervised assessments are necessary for acquiring real-world mobility data [34][35]. This is especially true, because gait characteristics from shorter walking bouts during daily living appear to be more informative about the disability level than longer bouts that are typically applied in laboratory-based tests [36]. This reinforces the need to further collect motion sensor data in the free-living setting. With evolving technology, it may be possible in the future to collect data continuously without the need for researchers to only collect data at selected periods.
3.2. Smart Systems for the Integration of Researchers, Clinicians, and Persons with MS
Smart systems for the ongoing monitoring of physical behavior among those with MS are essential for establishing and using biomarkers for the early detection of disease progression, and consequently, for effective and timely interventions for disease management [37]. These smart systems could record real-world accelerometer and IMUs data continuously, with minimal burden for the end-users [8]. Additionally, smart systems should meet the demands of researchers, clinicians, and end-users (persons with MS), and allow for a more integrated and inclusive disease management approach.
In a clinical perspective, the continuous monitoring of free-living walking and physical functions may provide clinicians with important ecological information on individuals who need immediate attention to prevent the worsening of their mobility status, as well as their disease progression. Conversely, researchers need data on the continuous monitoring of free-living physical behavior in order to develop more accurate methods/algorithms for predicting mobility and physical function outcomes, as well as disease progression. This can in turn be used by health professionals to improve clinical practice and disease management in MS by improving decision making, time of decisions, and strategies for mitigating or reversing deterioration in mobility, physical function, and disease course.
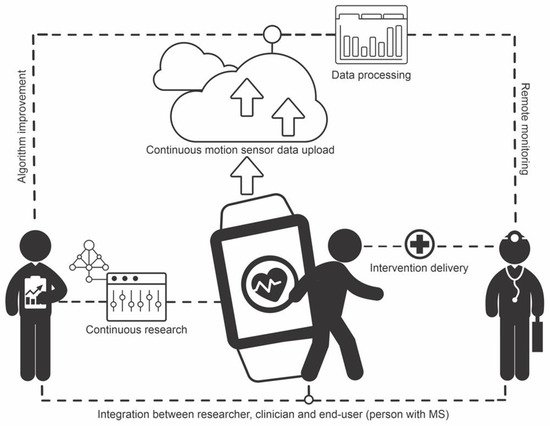
The integration of researchers, clinicians, and MS may be accomplished with digital cloud storage and computing technology, whereby motion sensor data for persons with MS are constantly collected, uploaded to the cloud, and processed remotely via algorithms implemented in servers, with outcomes and alerts being automatically sent to researchers and clinicians. Researchers may use the data to continuously improve the algorithms, whereas clinicians may use the data to improve clinical practice, providing personalized medicine to patients. Ultimately, this circle of constant feedback leads to better care for those with MS. Figure 1 illustrates the structure of such a system.

Figure 1. Illustration of the structure of a smart system for integration of researchers, clinicians, and people with multiple sclerosis. End-user motion sensor data are continuously uploaded to the cloud and processed via algorithms embedded in the cloud. Researchers use the data for algorithm improvement. Clinicians monitor patients remotely and deliver interventions as needed.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph191811839
References
- Sasaki, J.E.; Da Silva, K.S.; Da Costa, B.G.G.; John, D. Measurement of Physical Activity Using Accelerometers. In Computer-Assisted and Web-Based Innovations in Psychology, Special Education, and Health; Academic Press: Cambrige, MA, USA, 2016; pp. 33–60.
- Block, V.A.J.; Pitsch, E.; Tahir, P.; Cree, B.A.C.; Allen, D.D.; Gelfand, J.M. Remote Physical Activity Monitoring in Neurological Disease: A Systematic Review. PLoS ONE 2016, 11, e0154335.
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and Lifestyle Physical Activity Recommendations for People with Multiple Sclerosis throughout the Disease Course. Mult. Scler. Houndmills Basingstoke Engl. 2020, 26, 1459–1469.
- Murray, T.J. Multiple Sclerosis: The History of a Disease; Demos Medical Publishing: New York, NY, USA, 2005.
- Bradshaw, M.J.; Farrow, S.; Motl, R.W.; Chitnis, T. Wearable Biosensors to Monitor Disability in Multiple Sclerosis. Neurol. Clin. Pract. 2017, 7, 354–362.
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131.
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too Much Sitting: The Population Health Science of Sedentary Behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113.
- Sasaki, J.E.; Sandroff, B.; Bamman, M.; Motl, R.W. Motion Sensors in Multiple Sclerosis: Narrative Review and Update of Applications. Expert Rev. Med. Devices 2017, 14, 891–900.
- Motl, R.W.; McAuley, E.; Snook, E.M.; Scott, J.A. Accuracy of Two Electronic Pedometers for Measuring Steps Taken under Controlled Conditions among Ambulatory Individuals with Multiple Sclerosis. Mult. Scler. J. 2005, 11, 343–345.
- Elsworth, C.; Dawes, H.; Winward, C.; Howells, K.; Collett, J.; Dennis, A.; Sackley, C.; Wade, D. Pedometer Step Counts in Individuals with Neurological Conditions. Clin. Rehabil. 2009, 23, 171–175.
- Motl, R.W.; Snook, E.M.; Agiovlasitis, S. Does an Accelerometer Accurately Measure Steps Taken under Controlled Conditions in Adults with Mild Multiple Sclerosis? Disabil. Health J. 2011, 4, 52–57.
- Crouter, S.E.; Schneider, P.L.; Bassett, D.R. Spring-Levered versus Piezo-Electric Pedometer Accuracy in Overweight and Obese Adults. Med. Sci. Sports Exerc. 2005, 37, 1673–1679.
- Sandroff, B.M.; Motl, R.W.; Pilutti, L.A.; Learmonth, Y.C.; Ensari, I.; Dlugonski, D.; Klaren, R.E.; Balantrapu, S.; Riskin, B.J. Accuracy of StepWatchTM and ActiGraph Accelerometers for Measuring Steps Taken among Persons with Multiple Sclerosis. PLoS ONE 2014, 9, e93511.
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and Comparison of ActiGraph Activity Monitors. J. Sci. Med. Sport Sports Med. Aust. 2011, 14, 411–416.
- Sandroff, B.M.; Riskin, B.J.; Agiovlasitis, S.; Motl, R.W. Accelerometer Cut-Points Derived during over-Ground Walking in Persons with Mild, Moderate, and Severe Multiple Sclerosis. J. Neurol. Sci. 2014, 340, 50–57.
- Sandroff, B.M.; Klaren, R.E.; Pilutti, L.A.; Motl, R.W. Oxygen Cost of Walking in Persons with Multiple Sclerosis: Disability Matters, but Why? Mult. Scler. Int. 2014, 2014, 162765.
- Motl, R.W.; Snook, E.M.; Agiovlasitis, S.; Suh, Y. Calibration of Accelerometer Output for Ambulatory Adults with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2009, 90, 1778–1784.
- Sandroff, B.M.; Motl, R.W.; Suh, Y. Accelerometer Output and Its Association with Energy Expenditure in Persons with Multiple Sclerosis. J. Rehabil. Res. Dev. 2012, 49, 467–475.
- Motl, R.W.; Learmonth, Y.C.; Pilutti, L.A.; Gappmaier, E.; Coote, S. Top 10 Research Questions Related to Physical Activity and Multiple Sclerosis. Res. Q. Exerc. Sport 2015, 86, 117–129.
- Suh, Y.; Weikert, M.; Dlugonski, D.; Sandroff, B.; Motl, R.W. Social Cognitive Correlates of Physical Activity: Findings from a Cross-Sectional Study of Adults with Relapsing-Remitting Multiple Sclerosis. J. Phys. Act. Health 2011, 8, 626–635.
- Sandroff, B.M.; Dlugonski, D.; Weikert, M.; Suh, Y.; Balantrapu, S.; Motl, R.W. Physical Activity and Multiple Sclerosis: New Insights Regarding Inactivity. Acta Neurol. Scand. 2012, 126, 256–262.
- Klaren, R.E.; Sasaki, J.E.; McAuley, E.; Motl, R.W. Patterns and Predictors of Change in Moderate-to-Vigorous Physical Activity Over Time in Multiple Sclerosis. J. Phys. Act. Health 2017, 14, 183–188.
- Wójcicki, T.R.; Roberts, S.A.; Learmonth, Y.C.; Hubbard, E.A.; Kinnett-Hopkins, D.; Motl, R.W.; McAuley, E. Improving Physical Functional and Quality of Life in Older Adults with Multiple Sclerosis via a DVD-Delivered Exercise Intervention: A Study Protocol. BMJ Open 2014, 4, e006250.
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Breaks in Sedentary Time: Beneficial Associations with Metabolic Risk. Diabetes Care 2008, 31, 661–666.
- Coulter, E.H.; Miller, L.; McCorkell, S.; McGuire, C.; Algie, K.; Freeman, J.; Weller, B.; Mattison, P.G.; McConnachie, A.; Wu, O.; et al. Validity of the ActivPAL3 Activity Monitor in People Moderately Affected by Multiple Sclerosis. Med. Eng. Phys. 2017, 45, 78–82.
- Ezeugwu, V.; Klaren, R.E.; Hubbard, A.E.; Manns, P.T.; Motl, R.W. Mobility Disability and the Pattern of Accelerometer-Derived Sedentary and Physical Activity Behaviors in People with Multiple Sclerosis. Prev. Med. Rep. 2015, 2, 241–246.
- Klaren, R.E.; Sebastiao, E.; Chiu, C.-Y.; Kinnett-Hopkins, D.; McAuley, E.; Motl, R.W. Levels and Rates of Physical Activity in Older Adults with Multiple Sclerosis. Aging Dis. 2016, 7, 278–284.
- Bollaert, R.E.; Motl, R.W. Physical and Cognitive Functions, Physical Activity, and Sedentary Behavior in Older Adults With Multiple Sclerosis. J. Geriatr. Phys. Ther. 2019, 42, 304–312.
- Brocklebank, L.A.; Falconer, C.L.; Page, A.S.; Perry, R.; Cooper, A.R. Accelerometer-Measured Sedentary Time and Cardiometabolic Biomarkers: A Systematic Review. Prev. Med. 2015, 76, 92–102.
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.H.; Owen, N. Sedentary Time and Cardio-Metabolic Biomarkers in US Adults: NHANES 2003–06. Eur. Heart J. 2011, 32, 590–597.
- Motl, R.W.; Sosnoff, J.J.; Dlugonski, D.; Suh, Y.; Goldman, M. Does a Waist-Worn Accelerometer Capture Intra- and Inter-Person Variation in Walking Behavior among Persons with Multiple Sclerosis? Med. Eng. Phys. 2010, 32, 1224–1228.
- Weikert, M.; Suh, Y.; Lane, A.; Sandroff, B.; Dlugonski, D.; Fernhall, B.; Motl, R.W. Accelerometry Is Associated with Walking Mobility, Not Physical Activity, in Persons with Multiple Sclerosis. Med. Eng. Phys. 2012, 34, 590–597.
- Cavanaugh, J.T.; Gappmaier, V.O.; Dibble, L.E.; Gappmaier, E. Ambulatory Activity in Individuals with Multiple Sclerosis. J. Neurol. Phys. Ther. JNPT 2011, 35, 26–33.
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-Term Unsupervised Mobility Assessment in Movement Disorders. Lancet Neurol. 2020, 19, 462–470.
- Kluge, F.; Din, S.D.; Cereatti, A.; Gaßner, H.; Hansen, C.; Helbostad, J.L.; Klucken, J.; Küderle, A.; Müller, A.; Rochester, L.; et al. Consensus Based Framework for Digital Mobility Monitoring. PLoS ONE 2021, 16, e0256541.
- Storm, F.A.; Nair, K.P.S.; Clarke, A.J.; der Meulen, J.M.V.; Mazzà, C. Free-Living and Laboratory Gait Characteristics in Patients with Multiple Sclerosis. PLoS ONE 2018, 13, e0196463.
- Stavropoulos, T.G.; Meditskos, G.; Papagiannopoulos, S.; Kompatsiaris, I. EHealth4MS: Problem Detection from Wearable Activity Trackers to Support the Care of Multiple Sclerosis. In Ambient Intelligence–Software and Applications; Novais, P., Vercelli, G., Larriba-Pey, J.L., Herrera, F., Chamoso, P., Eds.; Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2021; Volume 1239, pp. 3–12.
This entry is offline, you can click here to edit this entry!
