Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Horticulture
The occurrence of polyploidy in plants was discovered about a century ago. Because of the widespread occurrence of polyploids in wild and cultivated plants, it is important for plant breeders and evolutionary biologists. In the past, antimitotic reagents-induced polyploids have not directly contributed to crop improvement.
- cytogenetics
- stress
- polyploidy
- fluorescent in situ hybridization
1. Role of Polyploidy in Modern Plant Breeding
Plant breeders modify crop traits using multiple tools, including polyploidization, to satisfy market demand. This technique creates intense phenotypes and high vigor, making it one of the most potent crop enhancement methods [38]. In addition, some plants have a specific demand for their specific traits, such as seedless fruits in grapevine and banana, which can be achieved through polyploidization [39,40]. Polyploidy results in higher heterozygosity and genome redundancy that are considered advantageous for improving crop plants over conventional plant breeding tools [41,42].
Interspecific hybridization helps to increase the diversity of crops and helps them adapt to new environments [43]. For example, Allopolyploid Triticale is a manufactured crop developed by crossing hexaploid bread wheat and rye to achieve specific goals (e.g., high yield, grain quality, less disease, and stress tolerance) [44,45]. In addition, bridge hybridization is done to transfer genes from one ploidy stage to another if a direct crossover is not feasible. Creation of diversity is one of the most important tasks to develop a crop variety where polyploidy has the efficacy to enhance crop diversity [46,47].
Polyploidy is common in newly domesticated crops [48]. In many cultivated crops, polyploidy has been observed in the speciation process and is now commonly used to create new species selected for features [3]. Unreduced gametes result in plant polyploidy and are used in crop breeding. Polyploidy increases the chromosome number, which helps plants tolerate the mutation by allelic modifications [49,50]. Chromosome deletion-related polyploidy breeding and substitution can produce targeted traits. We have seen many examples of successful cultivation influenced by polyploidy breeding. For example, seedless triploid watermelons, tetraploid red clovers, ryegrass, rye, and many ornamental plants have been developed or improved using polyploid breeding [17,51].
In summary, the main benefits of polyploidy are related to improving the use of heterozygosity. It buffers the effect of gene redundancy in mutations and, in some cases, facilitates reproduction by self-fertilization or asexual means [52]. It has a significant influence on farmers and food security issues.
2. Induction of Polyploids
The recurrence and frequency of polyploidization in plant species make polyploidization an influential research area [53] in which a major step is to select traits in plants [54]. The occurrence of polyploidy in plants was discovered about a century ago. Because of the widespread occurrence of polyploids in wild and cultivated plants, it is important for plant breeders and evolutionary biologists. In the past, antimitotic reagents-induced polyploids have not directly contributed to crop improvement. On the other hand, sexual polyploids (unreduced 2n gametes) are more relevant for crop improvement in many cases. Two pathways cause polyploids: mitotic polyploidization and meiotic polyploidization [55].
Mitotic polyploidization depends on doubling somatic tissue where homoeologous chromosome recombination occurs [56]. The first mitotic polyploidization was introduced in 1930 [57]. This activation polyploidization was tested on plants in vitro [55]. Colchicine, oryzalin, trifluralin, amiprophos-methyl, N2O gas treatment, and caffeine have recently been used as antimitotic reagents [17]. Colchicine is an alkaloid from wild meadow saffron and was the most used as an antimitotic reagent. Oryzalin is a potent herbicide from the Dow AgroScience, USA toluidine chemical band [17,58]. Wetting roots or auxiliary buds or shoots with a colchicine solution of a specific concentration and duration resulted in the successful development of polyploids in many crop species [57,59], as shown in Figure 1. Previous studies successfully applied in vitro chromosomes doubling of colchicine and oryzalin for starch, fodder beet, ryegrass, oriental melon, watermelon, and red clover [17,60].
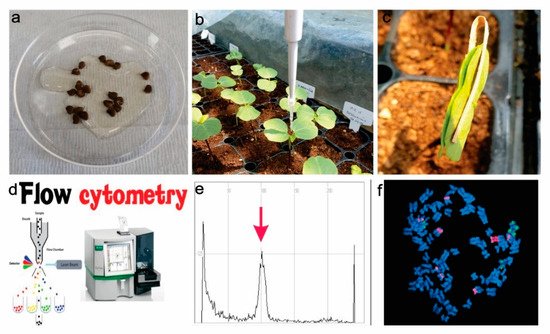
Figure 1. Mechanism of in vivo polyploidization; (a). Seeds soaking (6–24) hours with colchicine (0.01–0.2)%, (b). Colchicine treatment (10–20 µL) 10 days in the young leaves, (c). Leaves binding with clips for maximum chemical attachment, (d). Flow cytometry analysis for ploidy level assessment, (e). Ploidy level assessment by a histogram, (f). Hibiscus ploidy assessment using chromosome number; and 5S rDNA (green) and 18 rDNA (red) signals.
In vitro polyploidization showed better performance than the success rate of in vivo polyploidization in sugar and fodder beet, ryegrass, and red clover [55,59]. Table 1 provides a list of crops, vegetables, and ornamental and medicinal plants treated with chromosome antimitotic agents for chromosome duplication using different methods and protocols.
Table 1. Commonly used methods for polyploidization in vitro.
| Plants | Treatment | Most Successful Method | References |
|---|---|---|---|
| Vegetables | |||
| Allium | Callus | Colchicine 2.5 mM, 1/2 days | [60] |
| Citrullus lanatus | Germinating seedlings | 2,6-Dinitroaniline 65.5 µM, 24 h | [61] |
| Manihot esculenta | Axillary node cuttings | Colchicine 5 mM, 48 h | [62] |
| Smallanthus songifolius | Nodal segments | Oryzalin 25 µM, 8–48 h | [63] |
| Ornamentals | |||
| Buddleja | Nodal sections | Oryzalin 25 µM, 3 days | [64] |
| Dieffenbachia | Shoot clumps | Colchicine 1.25 mM, 24 h | [65] |
| Dracaena deremensis | Callus | Oryzalin 144.5 µM, 48 h | [66] |
| Hypericum | Callus | Oryzalin 30 µM, 3–9 days | [67] |
| Lagerstroemia indica | Nodal buds | Colchicine 750 µM 24 h | [68] |
| Rhododendron | Micro-shoots | Oryzalin 150 µM, 24 h | [69] |
| Rosa | Shoots tips, nodal sections | Oryzalin 5 µM, 1 day | [70] |
| Rosa rugosa | 2 or 10mm nodes | Oryzalin 2.5 µM, 48 h | [71] |
| Syringa | Nodal sections | Colchicine 0.05–0.25 mM, 1–2 Days | [72] |
| Alocasia | Shoot tips | Oryzalin 289 µM, 24 h | [73] |
| Alstroemeria | Plantlets | Colchicine 5–15 mM, 6–24 h | [74] |
| Cattleya | PLB | Colchicine 1.25 mM, 8 days | [75] |
| Cyclamen | Tuber segments | Colchicine 0.25 mM, 4 days | [76] |
| Lilium longiflorum | Scale | Surflan (0.1 mM oryzalin), 3 h | [77] |
| Tulipa gesneriana | Flower stem dices | Oryzalin 2.88–120 µM, 2–24 h | [78] |
| Watsonia lepida | Shoots | Oryzalin 120 µM, 24 h | [79] |
| Zantedeschia | Shoot cultures | Colchicine 1.25 mM, 1–4 days | [80] |
| Aromatic, medicinal plants | |||
| Astragalus membranaceus | Apical buds | Colchicine 5 mM, 36 h | [81] |
| Bixa orellana | Cotyledonary nodes from seedlings | Oryzalin 15 µM, 15 days | [82] |
| Colophospermum mopane | Seeds | Colchicine 2.5 mM, 48 h | [83] |
| Dioscorea zingiberensis | Apical buds | Colchicine 3.75 mM, 24 h | [53] |
| Humulus lupulus | Apical buds | Colchicine 1.25 mM, 48 h | [84] |
| Zingiber officinale | Shoot tips | Colchicine 5mM, 8 days | [85] |
Meiotic polyploidization produces 2n gametes due to the incomplete division of chromosomes [86]. Polyploids that originate through the functioning of 2n gametes are called sexual polyploids, and their usefulness for crop improvement has been demonstrated in potato, alfalfa, and red clover. Introgression can be accomplished by recombination due to genetic crossing-over between alien chromosomes as well as the addition of alien chromosomes in the case of sexual polyploidization in allopolyploids, which is exceedingly difficult or unlikely in the case of colchicine or oryzalin induced allopolyploids. This deviation can occur in plants with normal chromosome pairing as well as in those with disturbed chromosome pairing such as homoeologous recombination of meiotic replication that was seen in Alstroemeria [87], Lilium [88] and Gasteria lutzii × Aloe aristate [89]. The process leading to the formation of 2n gamete is called meiotic nuclear restitution during micro- or megasporogenesis. Depending on the meiotic stage at which nuclear restitution occurs, different restitution mechanisms have been recognized, such as first division restitution (FDR), second division restitution (SDR) [90], and novel intermediate meiosis restitution [88]. In FDR, the non-sister chromatids are heterozygous from the centromere to the first convergence point, while preserving heterozygosity in both parents [91]. In SDR, the two sister chromatids are homozygous between the centromere and the first crossover point, and the resulting gametes have lowered heterozygosity levels compared to the parents [92]. In some cases, 2n gametes restitution cannot be classified as FDR or SDR; the word “indeterminate meiotic restitution” (IMR) has been coined to describe it [88]. Furthermore, IMR might be a widespread occurrence in allotriploids, where both bivalents and univalents are most produced.
3. Effect of Polyploidization at the Morphological and Molecular Level
Polyploidization results in morphological changes in plants due to whole genome duplication, changes in chromosomal structure, nuclear enlargement along with gene dosage and epigenetic consequences, as well as an increased number of larger cells [18,110,111,112,113,114]. Further, due to the changes in different levels, several morphological traits such as plant height, root length and number, leaf number, area and size, pollen size and number, stomata number and size, and flowers and fruits number and size as summarized in Table 2.
Table 2. Effect of polyploidization on plant morphology and yield attributes.
| Induced Polyploid | Effect (Increased/Decreased) |
References |
|---|---|---|
| Plant height | Increase | [52,54,115] |
| Root length and number | Increase | [86,116] |
| Number of leaves/plants | Increase/decrease | [117] |
| Leaf area | Increase/decrease | [17,118] |
| Leaf size | Increase | [17,112] |
| Stomata number/leaf | Decrease | [17,119,120] |
| Stomata size | Increase | [17,115,119] |
| Flower size, number | Increase | [52,112,117,118,119,120,121,122,123,124,125] |
| Pollen size | Increase | [123] |
| Fruit size, number | Increase | [98,116,124] |
| Seed size | Increase | [98,116] |
| Seeds/fruit | Decrease | [17,39] |
With the increase of ploidy level, the plant height, width and length of flower, flower size, and the number of internodes in dendrobium increased [17,126,127,128]. Polyploidization affects the floral traits such as flowering time, flower diameter, shape, size, and color, as well as different parts of flowers in kiwifruit and salvia [9,129,130,131,132,133,134,135,136,137]. These shreds of evidence suggest that polyploidy can be applied in plant breeding by targeting the flower size, shape, color, modifications in size, and the number of floral parts. Fruit size and fruits number, along with other fruit characteristics such as fruit weight, fruit peel, flesh weight and seed number are affected by the increase of ploidy number [130,138,139,140,141,142,143,144]. Due to variations in cell size and chromosome size (Figure 5), polyploidy changes the characters of the leaf [145,146,147,148,149,150,151,152,153]. Stomata number, density, size, and area are the important traits of leaves that are affected by the change of ploidy number [154,155], and this effect (Figure 5) has been observed in citrus [120].

Figure 5. Leaf morphology and stomata size of watermelon induced by oryzalin. (a). diploid leaf, (b). tetraploid leaf, (c). stomata of diploid, and (d). stomata of a tetraploid leaf, respectively. Scale bar= 10 μm [17].
Changes in fruits, leaves, flowers, and color can be considered from the application point of view. Targeted traits can be achieved along with higher variation with the changes of ploidy level. A change in ploidy level also affects molecular and gene expression. Changing the ploidy level due to changes in nuclear DNA, chromosome number, and structure can manipulate genetic diversity, genome replication, gene expression, and heterosis [156]. Changes in ploidy level affect DNA content and the number of chromosomes [157,158,159]. During replication, polyploidization often induces epigenetic changes such as transposon simulation and chromatin modification, as well as the extension or loss of chromosomal fragments. The polyploidization effect at the plant morphology, physiology, and molecular levels needs extensive research to reveal the mechanisms that will help plant breeders for directed modification and crop improvement.
This entry is adapted from the peer-reviewed paper 10.3390/plants11202684
This entry is offline, you can click here to edit this entry!
