Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Forestry
The cork layer present in all dicotyledonous plant species with radial growth is the result of the phellogen activity, a secondary meristem that produces phellem (cork) to the outside and phelloderm inwards. These three different tissues form the periderm, an efficient protective tissue working as a barrier against external factors such as environmental aggressions and pathogen attacks.
- cork
- exploitation
- phellem
- phellogen
- stress
1. Growth and Uses
The cork oak Quercus suber L. (Fagaceae) is an endemic tree of the Mediterranean basin growing mainly in Portugal, Spain, southern France and Italy, and northern Morocco. Cork oak stands, known as “montados” in Portugal and “dehesas” in Spain, are complex agro-silvo-pastoral systems managed by human labor to provide a habitat for a diverse wildlife and cultural ecosystems services. Management and exploitation of cork oak stands dates back to roman times and, throughout the centuries, Portugal alone has become the world leader of cork production (55% of world’s production) and cork transformation [1,2,3].
Cork possesses a set of properties, i.e., a low material density and an extremely low permeability to liquids and gases, it is biologically and chemically inert and mechanically elastic, and the conferring cork material provide high insulation and damping capacities [1]. Due to all properties of cork, it is used in a plethora of products such as sealants, agglomerates and composites that can be transformed into bottle stoppers, insulation and surfacing panels for construction and aeronautics, for pollutants absorbers, for clothing, fashion items and decorative furniture pieces [4,5]. Besides the specific uses for cork singled out, other compounds can also be extracted from bark, which are later used in medicine, construction, chemistry, clothing, energy and biofuels [6]. Cork has also been found to work as biosorbents for heavy metals [7,8], oils [9] and aromatic hydrocarbons [10].
Cork can be extracted from the tree as a single plank because the phellogen (the meristematic layer producing cork outwardly) forms a continuous cylinder layer all around the trunk. Cork oak debarking for cork exploitation can only occur during a short time window from June till July (highly dependent on the environmental conditions), when phellogen cells are fully active and the new cells produced still display thin and fragile cell walls. As far as industrial requirements are concerned, for cork quality, the first cork produced by the tree and harvested, also known as virgin cork (Figure 1a), cannot be used for high end products because it is a hard-rough cork, very irregular in cell/material density and thickness. After the first cork, each debarking occurs every 9-12 years allowing the tree, the time necessary to regrow the tree’s outer periderm. This cork, used by industry, is called reproduction cork and displays enough thickness to allow punching natural cork stoppers (planks must be at least 27 mm thick) (Figure 1b). Regardless, all cork removed from the tree can be exploited commercially as raw material for final products as agglomerates. Only second reproduction cork and following harvests named “amadia” cork meets the quality standards necessary for stopper production demanded by wine companies [11] (Figure 1b). Stopper bottles are the product with the higher industrial revenue; however, the amount of available good-quality cork needed is becoming scarcer in the field, compromising cork manufacturing goals. For several years now, the transformation industry is developing new products made from lower quality cork and leftovers from stopper production, reducing the dependency on the wine sector [2,12]. Furthermore, in cork exploitation, there is a growing interest in understanding periderm development to improve plant resilience and as sinks for CO2 sequestration [13].

Figure 1. The cork layer of Quercus suber. (a) Virgin cork. This cork oak has never been debarked is still displaying its first cork layer. (b,c) Different quality types of amadia cork. (b) Cork plank thick enough and with very few discontinuities allowing stopper punching (red dots circle). It is possible to distinguish the growth rings (black arrow). (c) Example of a reduced-quality cork plank, thin in thickness and intersected by numerous lenticular channels (black arrowhead). White arrows indicate the growth direction from the surface in contact with the trunk and where the phellogen is (also known as cork belly) towards the surface in contact with the environment. In—cork plant inside; out—outermost cork plank surface.
2. Biology and Adaptation
Several herbaceous species display a limited secondary growth, produced by interfascicular and fascicular meristems, therefore lacking phellogen and a periderm. Most herbaceous or woody plants display secondary growth of plant organs, which confers widening and is accomplished through the activation of two secondary meristems; the vascular cambium and the phellogen (cork cambium). The increase in diameter reflects the periclinal divisions on these two cambiums, producing derivatives towards both ends with further distinct specifications. The vascular cambium produces xylem inwardly and phloem outwardly resulting in stem and root thickening. This vascular growth forces the primary epidermis to break down with concomitant development of a complete periderm, a protective tissue of mature organs [15]. The periderm is composed of the phellogen, the phelloderm (produced by the phellogen inwards) and the phellem (cork) present outward of the phellogen [1] (Figure 2j,k). In most woody species growing in temperate climates, the first periderm is replaced by a new functional periderm a few years after being formed. This way, bark begins to accumulate on the outside comprising layers of dead periderms and remaining non-functional phloem tissue between them. This structure is called rhytidome [16]. One exception to this bark development can be found in Quercus suber which displays a single periderm that grows continuously. Such continuous growth of the phellem gives rise to cork rings that can be clearly distinguished because spring forming cells have thinner walls and a larger diameter compared to the cells formed later in the season [17] (Figure 1b).
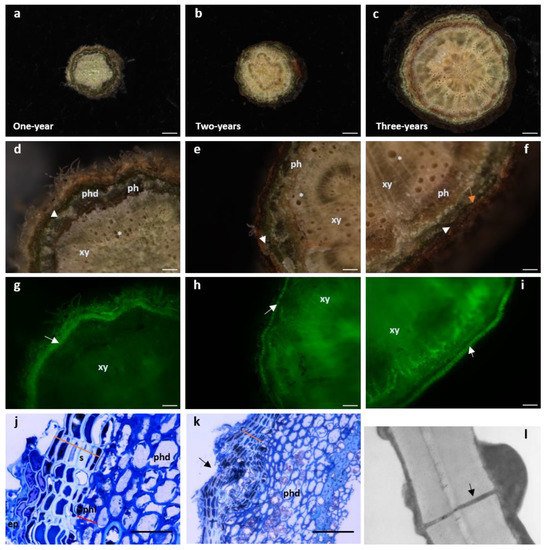
Figure 2. Cork develops as early as one-year stems in Quercus suber. (a–i) Cross-sectional stereomicroscopic images of one-, two- and three-year-old stems. (Scale bars: (a,b) 100 µm, c 500 µm). (d–f) Details of the xylem with empty tracheids (*). Moving outwardly, one finds the phloem (dark layer) surrounded by the periderm comprising the phelloderm (orange arrow -phd), a thin layer of phellogenic cells (white arrowhead) and several rows of suber cells. The most external layer is the remaining epidermis. (Scale bars: (d,e) 100 µm, f 500 µm). (g–i) Cork cells exhibit autofluorescence when excited with UV light [18]. The stems observed in d-f were excited with UV using a stereoscope filter for “Lumar01” (BP 365/12; LP397) after which, the suberin layer is clearly distinguishable (white arrow). In older stems, as xylem cells mature, autofluorescence can be observed since lignin also possesses some autofluorescence properties under UV light. (j,k) Light microscopy of one-year stem cross sections stained with toluidine blue. (Scale bars: (g,h) 100 µm, i 500 µm). (j) Some remaining epidermis (ep) is still present and right below, six to seven cell cork layers with cells displaying a hyaline cell wall appearance and filled with electrodense material (phenolic compounds) (orange line) can be observed. Inwardly and adjacent to suberin cells, is the phellogen composed of only one to two cells (red line). Cells are filled with cytoplasm and cell walls do not present a secondary thickening. Right below is the phelloderm with the characteristic round-shaped parenchymatous cells. (Scale bar: 10 µm). (k) Cross section showing a lenticel forming in a one-year stem (black arrow). The disorganized division of the meristematic cells inside the lenticel structure is starting to push the suberin layer outwards leading to a complete rupture of this layer to form an aperture that allows gas exchanges. (Scale bar: 40 µm). (l) Transmission electron microscopy image of amadia cork cell wall clearly showing a plasmodesmata (black arrow) crossing both suberized cell walls. It is possible to observe cytoplasmic deposition in the inner part of the cell, with thickening appearing on both sides of the plasmodesmata channel (amp. 20,000×). ep—epidermis; ph—phloem; phd—phelloderm; phl—phellogen; s—suberin; xy—xylem.
The activity of the phellogen begins in April and continues to be active till the end of October [18]. During these months, growth is not uniform. In early season (April–July), cells are formed faster, thus display thinner walls and larger lumina diameter than cells growing later in the season (August–October). Such difference in cell anatomy permits distinguishing cork rings in a similar fashion to tree-rings (Figure 1b). Weather plays a role in cork-ring growth with a higher growth resisted in rainy years with moderate-low temperatures [19]. In fact, even outside of growing season (November–December), precipitation positively influences cork growth [20] as well as moderate temperatures during the summer [21]. However, it has been verified that high summer temperatures negatively influenced cork production [13]. The constrains that weather pressure exert on cork development is of great worry for cork producers and the cork industry.
Since suberized cells are impervious, water vapor and the gases CO2 and O2 are regulated through lenticels. Lenticels are aerenchymatous structures composed mainly of active meristematic cells interspersing the phellem layer allowing gaseous exchange between the tree and the environment [24] (Figure 2k). Lenticels, unlike the neighboring suberized cells lack suberin but are essentially composed of lignin [1]. Lenticels arise from previous structures involved in gases exchange, the stomata, which are present in the epidermis and are genetically determined. For the cork industry, the presence of a high number of lenticels downgrades the cork quality, thus bringing its price down for producers (Figure 1c). Therefore, understanding the ontogeny of lenticels and the reason why some trees display a much higher number of these structures is of great importance to cork production.
3. Chemical Composition
The characteristic cork properties are a direct reflection of the cell wall’s chemical composition and structure. Cork’s chemical nature dictates its function as a protective layer of the internal tree’s tissues against environmental aggression, which is achieved by its main cell wall component, suberin. Other important cork cell wall components are lignin, the polysaccharide cellulose and the hemicelluloses and polar and non-polar extractives [1]. Cork cells present a wide chemical variation concerning suberin contents (23.1% to 54.2%) and lignin (17.1–36.4%) with the ratio suberin-to-lignin playing a pivotal role in physical cork properties such as compression [25,26].
Comparing the chemical composition of Q. suber’s cork, phloem and wood tissues, cork cells are the only ones with suberin on its cell walls (comprising 42.3%). Phloem on the other hand, shows a higher degree of lignification in comparison to wood and have less polysaccharides [27].
Suberin is made up of two domains: an aliphatic zone and an aromatic zone that includes ferulic acid [28]. One of the most recent accounts of suberin’s ferulic acid content was of 2.7% [29]. The polymeric aliphatic macromolecule is composed of two types of monomers: glycerol and long chain fatty acids and alcohols, whose hydroxyl and carboxylic groups are linked by ester bonds [30]. The monomer glycerol represents 40.8% of suberin and the long-chain monomers comprising mainly α-ω-diacides at 36.4% [31]. The fatty acids present chain lengths that can vary from C16 to C30, esterified to glycerol and cross-esterified [32]. At suberin’s macromolecular structural level, the polyester aliphatic structure is extensively linked to aromatic moieties [33] conferring the secondary cell wall, the appearance of lamellate structure as seen by transmission electron microscopy (TEM) [34]. These lamellae show alternate opaque and translucent contrasting structures, with translucent lamellae showing a regular thickness of ca. 30 Å and the opaque lamellae varying in thickness of ca. 70 to 100 Å. Between 30 and 60 lamellae have been counted in the suberized cell walls of cork of different species [34]. Recent work comparing the ultrastructural analysis of secondary cell walls in Quercus suber, Quercus cerris, Calotropis procera and Solanum tuberosum found that the lamellar structures with alternating dark and light bands were present in suberized cells of potato tuber periderm and Calotropis bark, whereas the cork cells of Q. cerris and Q. suber lacked defined lamellae [35]. Such findings led to the hypothesis that the chemical composition of suberin, which differs between species, may play a role in the cell wall topochemistry due to different spatial development of the suberin macromolecule [35].
The second most important cork cell wall component is lignin which confers strength to the cell. The remaining cell wall polysaccharides are cellulose and hemicellulose accounting for 20% of the cork’s cell wall structural components [36,37]. Lignin is present in most plants’ secondary tissues. It is a polymer comprising three different types of phenyl propane monomers, conferring an aromatic nature to lignin. The monomers ρ-coumaryl, coniferyl and sinapyl alcohols are linked through enzymatic phenoxy radical formation [1,30]. Lignin is also linked to ferulic acid by ester bonds accounting for 3% [29].
Non-structural components, soluble in different solvents, are also present in cork, such as lipophilic extractives that include fatty acids and alcohols, sterols and terpenes and phenolic compounds [6,38]. Ash is an inorganic material found in percentages ranging from 1% to 2% and is the end result upon total combustion. Amongst all ash components, calcium, phosphorous, sodium, potassium and magnesium are the minerals present in the highest concentrations [37,39]. In sum, in cork cells, the composition of the secondary cell wall depositions that takes place in the interior of the cell is of about 42% suberin, 22% of lignin, 19% polysaccharides and 16% of extractives [25]. The extractive portion is a myriad of low to medium molecular weight molecules and includes: n-alkanes, n-alkanols, waxes, triterpenes, fatty acids, glycerides, sterols, phenols and polyphenols that can be sorted in two main groups; aliphatic and phenolic [1]. Suberin, requires the presence of a lignin-like polymerized aromatic domain to adhere to the cell wall [40], whereas waxes are aliphatic compounds that do not covalently link to the cell wall [41]. Suberization is a fast process which can be visualized as soon as phellogen cells grow giving rise to a differentiated phellem (Figure 2j) [42]. Phellem development begins as soon as one-year stems grow (Figure 2a,d,g) and become thicker as the tree ages (Figure 2b,c,e,f,h,i). This can be detected on the roots and the aerial parts of the tree [43].
Polyphenolics are another group of extractives found in cork cells that are much less studied, with more heterogenous composition among phellems, which include simple phenols and/or polymeric phenols such as tannins. Out of the few studies on polyphenols in cork, Pinheiro et al. (2019) [44] were able to establish a comparison of phenolic compositions between cork samples of higher and lower quality findings that in cork of higher quality, aromatic phenylpropanoid components were incorporated into the cell wall in larger amounts than hydrolysable tannins. This work was carried out in parallel with a transcriptomic analysis using the same cork samples. Here, it was possible to see that during the development of cork of superior and inferior quality, and in the former, the shikimate pathway shifts towards the synthesis of cell wall-bound phenolics. In the latter, phellogenic cells invested more into the biosynthesis of soluble phenolics, especially hydrolysable tannins, displaying a higher reducing capacity (40% more than in cork of higher quality) [44,45].
This entry is adapted from the peer-reviewed paper 10.3390/plants11202671
This entry is offline, you can click here to edit this entry!
