Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Radiotherapy remains an effective conventional method of treatment for patients with cancer. However, the clinical efficacy of radiotherapy is compromised by the development of radioresistance of the tumor cells during the treatment. The main goal of radiotherapy is to destroy or slow tumor growth by using high-energy radiation, such as X-rays, gamma (γ) rays, electrons, protons, neutrons, and carbon ions. The efficacy of killing is influenced by a number of factors, including the type of radiation, the total dose, the fractionation rate, and the targeted organs.
- cancer
- radiotherapy
- cell death
- radioresistance
- immunomodulating effects
1. Introduction
Globally, malignant tumors have become major conditions that significantly compromise human health and lives. According to the GLOBOCAN estimates from the International Agency for Research on Cancer, a total of 19.3 million new cases of cancer and 10 million deaths were expected to occur in 2020 [1]. Radiation therapy (RT) is one of the most important treatment strategies against malignant tumor cells. Approximately 50% of patients with cancer are estimated to receive RT as part of their treatment, and it has been reported that 40% of patients are cured by the therapy [2][3]. However, the success of radiotherapy is threatened by the emergence of radioresistance by cancer cells and the RT-induced damage to normal cells.
Currently, there is an increasing understanding of the radiobiological effects and mechanisms of tumor cells in response to RT. This is an important projection toward the development of new therapeutic strategies and the realization of optimal treatment outcomes in patients with cancer [4]. Although some progress has been made in the tumor radiobiological response, there is still a need to elucidate more of the molecular mechanisms and biological signatures of tumor cells after exposure to radiation.
2. RT-Induced Cancer Cell Death
The main goal of RT is to destroy or slow tumor growth by using high-energy radiation, such as X-rays, gamma (γ) rays, electrons, protons, neutrons, and carbon ions. The efficacy of killing is influenced by a number of factors, including the type of radiation, the total dose, the fractionation rate, and the targeted organs. It is noted that different tumors show different levels of sensitivity to RT. Previous studies have shown that the primary intracellular target of RT is DNA [5]. RT triggers DNA damage through the direct deposition of ionizing energy into the DNA or via the production of free radicals. Consequently, the damaged DNA is efficiently detected and repaired through homologous recombination (HR) or non-homologous end-joining (NHEJ) mechanisms. At present, NHEJ is the preferred pathway for repairing RT-induced DNA damage. A high radiation-associated deletion burden was associated with poor survival and may be able to predict the sensitivity of recurrent cancer after RT [6]. Conversely, improper or inefficient mechanisms of DNA repair induce different manners of cell death, including mitotic catastrophe, apoptosis, and senescence (Figure 1). Comprehensive reviews on the molecular mechanisms of these cell death phenotypes have previously been studied and published [7][8].
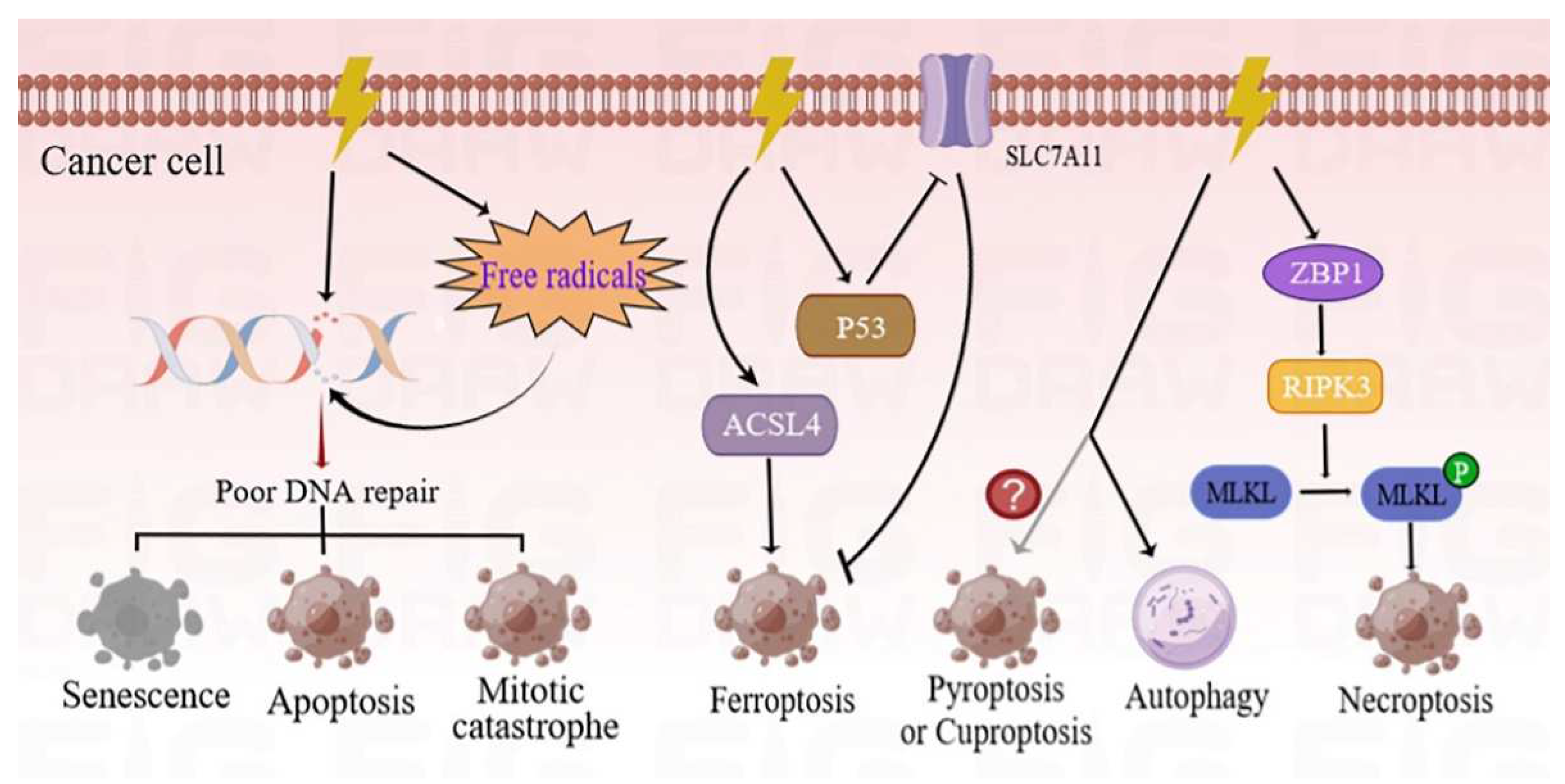
Figure 1. Different forms of cancer cell death programs induced by RT. RT can directly damage the DNA through production and deposition of ionizing energy or indirectly via free radicals. The DNA strands can be damaged in single-strand and double-strand breaks. If these DNA lesions cannot be properly repaired, tumor cells will initiate different types of death programs, including mitotic catastrophe, apoptosis, and senescence. Ionizing radiation (IR) can induce not only the expression of ACSL4 but also the activation of p53, and hence, results in elevated ferroptosis. The ZBP1-RIPK3-MLKL necroptotic cascade induces accumulation of cytoplasmic mtDNA in irradiated tumor cells, and consequently, activates an anti-tumor immune response. Conversely, autophagy is more inclined to cause cancer cell survival following IR treatment. However, there is a need for further studies to determine whether RT can induce pyroptosis or cuproptosis. ACSL4—acyl-CoA synthetase long-chain family member 4; p53—tumor protein p53; ZBP1—Z-DNA-binding protein 1; RIPK3—receptor-interacting serine/threonine kinase 3; MLKL—mixed-lineage kinase domain-like pseudokinase; mtDNA—mitochondrial DNA.
The modes of cancer cell death induced by RT in recent years are primarily summarized in the current entry. Among them, ferroptosis is an iron-dependent cell death strategy which is triggered by excessive lipid peroxidation. Notably, RT can induce ferroptosis by inducing the expression of acyl-CoA synthetase long-chain family member 4 (ACSL4) [9]. Simultaneously, the tumor protein p53 enhances RT-induced ferroptosis, partly by inhibiting the expression of solute carrier family 7 member 11 (SLC7A11) [10]. As a new form of immunogenic cell death program, necroptosis, which is regulated by Z-DNA-binding protein 1 (ZBP1)-mixed-lineage kinase domain-like pseudokinase (MLKL) signaling, has been found to improve the radiation-induced antitumor immunity of cells [11]. On the contrary, autophagy is more inclined to induce tumor resistance and is discussed in a subsequent section of this entry. However, there is a need for further investigation to determine whether RT is able to induce pyroptosis or cuproptosis.
In general, there is a dose–effect relationship between the radiation dose and tumor control rate (that is, the increase in radiation dose can increase the killing effect of tumor cells within a certain scope). Most of researchers' understanding of radiobiological principles is based on conventional photon radiotherapy (doses of 1.8–2.0 Gy per day) outcome studies. The most widely accepted model is the linear–quadratic (LQ) model. Currently, RT has entered the era of unconventional fractionated radiotherapy, including stereotactic body radiotherapy (SBRT), and FLASH RT. While the LQ model has been widely used for decades in radiation oncology, its applicability to hypofractionated RT remains controversial. Additional efforts are required to fully exploit the potential of these novel RT techniques to guide clinical interventions.
3. Molecular Mechanisms of Radioresistance in Tumor Cells
Radioresistance is a major impediment to therapeutic efficacy and results in tumor recurrence, as well as distant metastasis. Previous studies have recently reported on the mechanisms of radioresistance mediated by the tumor stroma [12][13]. The present entry mainly reviews the radioresponse of resistant cancer cells (Figure 2). Moreover, the post-radiation-exposure tumor-induced immunosuppression effects are also summarized in a subsequent section of the current entry.
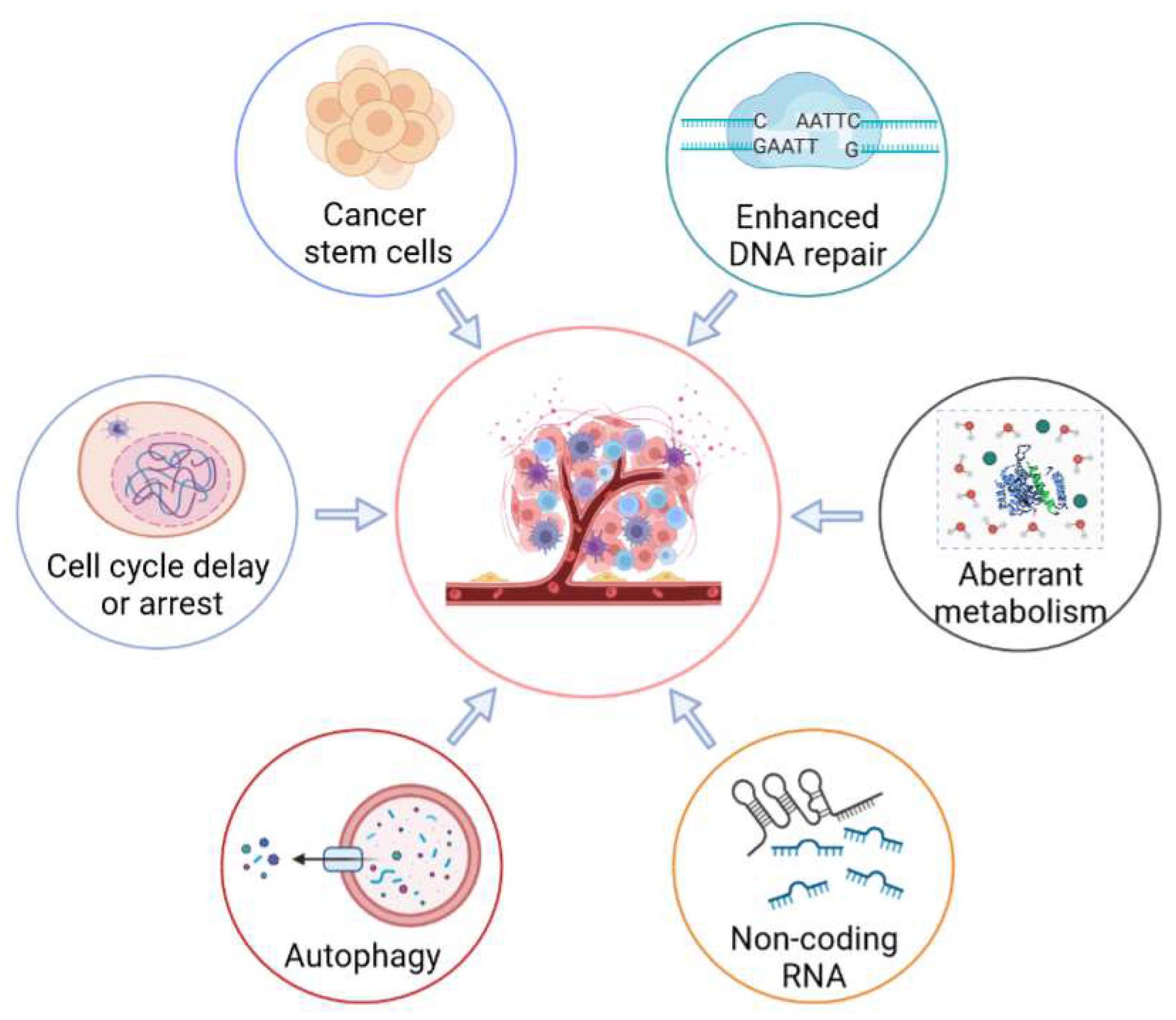
Figure 2. Molecular mechanisms of radioresistance mediated by tumor cells. Radioresistance of tumor cells is a major barrier to successful cancer treatment. In order to evade cell death, tumor cells have developed a variety of strategies, including enhancing DNA repair, activating cell-cycle checkpoints, regulating the self-renewal and differentiation of cancer stem cells, enhancing cellular metabolism, regulating the activities of autophagy, and regulating the expression of non-coding RNA.
3.1. Enhanced DNA-Repair Capability
RT kills tumor cells mainly by inducing double-strand DNA breaks. Therefore, an increased ability for DNA repair is strongly correlated with radioresistance in cancer cells. Serine proteinase inhibitor clade E member 2 (SERPINE2) promotes the repair potential of HR by activating the downstream repair protein RAD51 [14]. In a different study, the kinase ATM phosphorylates and stabilizes zinc finger E-box binding homeobox 1 (ZEB1). Then, ZEB1 binds to ubiquitin-specific peptidase 7 (USP7) and enhances its ability to deubiquitylate as well as stabilize the checkpoint kinase 1 [15]. In addition, pancreatic progenitor cell differentiation and proliferation factor (PPDPF), as well as Bromodomain-containing 4 (BRD4), can also enhance the repair capacity of tumor cells [16][17].
The deletion of leucine-rich repeat-containing protein 31 (LRRC31) can enhance DNA repair through genome-wide CRISPR library screening. This occurs by through regulation of the activity of DNA-PKcs and ATR-MSH2 signaling [18]. Previous studies have found that the repression of ubiquitin-conjugating enzyme E2O (UBE2O) or aurora kinase A (AURKA) can also promote radiation-induced DNA damage in lung cancer cells [19][20].
3.2. Cell-Cycle Checkpoint Activation
The integrity of DNA molecular structure and function is essential in the maintenance of the normal cellular function. When the DNA structure is damaged, the cell cycle of the affected tissue can be delayed or arrested to provide sufficient time for DNA repair. Therefore, tumor cells can enhance resistance to radiation by regulating cell-cycle arrest. For instance, cyclin K plays pivotal roles in the regulation of the responses that lead to DNA damage and control the G2/M checkpoint through modulation of the β-catenin/cyclin D1 axis [21]. A separate previous study has revealed that a positive feedback loop of the DNA-PK/AKT/GSK3β/cyclin D1 pathway can also activate the DNA-damage checkpoint [22]. Ubiquitin-conjugating enzyme E2T (UBE2T) interacts with and monoubiquitinates H2AX upon exposure to radiation, and hence, facilitates the activation of CHK1, as well as the cell-cycle arrest [23].
Topoisomerase IIβ-binding protein 1 (TopBP1) or Claspin is an important mediator in the checkpoint which increases the phosphorylation of CHK1 for the radioresistance of lung cancer cells [24]. In addition, RT can drive p21-activated kinase 1 (PAK1) to phosphorylate RAF1 on serine 338 and recruit checkpoint kinase 2 [25]. Notably, the HPV oncoproteins E6 and E7 promote the expression of the Ras-associated binding protein Rab12 to facilitate the arrest of G2/M [26]. Further, it has been shown that caspase-activated DNase (CAD) actively promotes self-inflicted DNA breaks, which leads to the arrest of the G2 phase in cancer cells, and hence, increases the survival of the cell after RT [27].
3.3. Cancer Stem Cells (CSCs)
Cancer stem cells (CSCs) represent a small population of cells (approximately 1~2%) in tumor tissue with characteristics of stem cells. Increasing evidence indicates that CSCs are closely related to the occurrence, treatment, prognosis, recurrence, and metastasis of tumors [28][29][30]. Fractionated irradiation promotes N-cadherin expression, which maintains the stemness of glioma stem cells by inhibiting Wnt/β-catenin signaling [31]. On the contrary, ribosomal S6 protein kinase 4 (RSK4) promotes CSC properties in esophageal squamous cell carcinoma (ESCC) by activating the Wnt/β-catenin pathway [32]. In colorectal cancer cells, JAK2/STAT3/CCND2 signaling contributes to cancer cell stemness and radioresistance [33]. In addition, it has been found that S100 calcium-binding protein A9 (S100A9) mediates the properties of CSCs in the brain microenvironment by activating the RAGE/NF-κB pathway [34]. It has been reported that with ultra-high doses of irradiation (~109 Gy/s), the CSCs may enhance lysosome-mediated autophagy and decrease pyroptosis and apoptosis, as well as necrosis [35].
Pluripotency transcription factors, such as octamer-binding transcription factor 4 (OCT4), SRY-box transcription factor 2 (SOX2), and Nanog homeobox (NANOG), play an important role in the regulation of the self-renewal and differentiation of CSCs. It has been noted that THO complex 2/5 can facilitate the mRNA export and translation of SOX2 and NANOG [36]. Similarly, RAD51AP1 regulates the self-renewal of CSCs in breast cancer cells [37]. Additionally, the upregulation of ALG3 promotes the glycosylation of transforming growth factor beta receptor 2 (TGFBR2). This later activates the TGF-β/Smad pathway, which further promotes the expression of OCT4, NANOG, and SOX2 [38]. Moreover, the positive-feedback activation of ROS/AKT signaling contributes to the enrichment of CSCs in nasopharyngeal carcinoma [39].
3.4. Aberrant Metabolism
Deregulating cellular metabolism is a hallmark of cancer, and hence, can regulate the resistance of cancer cells to therapy [40]. For instance, the high expression of glutamine synthetase can promote nucleotide metabolism for the efficient repair of damaged DNA, and hence, contributes to the resistance of tumor cells against radiation [41]. In lung cancer, KEAP1/NFE2L2 mutations depend on glutamine metabolism to decrease intracellular ROS levels [42]. In addition, purine metabolism has been found to protect glioblastoma from radiation by promoting the repair of damaged DNA [43]. It has also been reported that metabolic pathways which are regulated by nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADPH) can also potentiate radiation resistance in glioblastoma [44][45]. Moreover, oncostatin M receptor (OSMR) promotes oxidative phosphorylation by interacting with the NADH ubiquinone oxidoreductase core subunit S1/2 (NDUFS1/2) [46].
Recently, fatty acids have been found to be an alternative pivotal resource for energy, and thus, contribute to radiation-induced resistance by facilitating the oxidation of mitochondrial fatty acids (FAO) [47][48][49]. Notably, fatty acid metabolism can also enhance CD47-mediated anti-phagocytosis in glioblastoma multiforme. Mechanistically, acetyl-CoA, which is one of the main metabolites of FAO, upregulates the expression of CD47 by acetylating RelA K310 [47]. It is also notable that the crosstalk between lipid droplets and iron metabolism makes cancer cells more radioresistant [50]. In hepatocellular carcinoma (HCC) cells, the integration of glucose and cardiolipin anabolism, which is regulated by the mTORC1/HIF-1α/SREBP1 axis, promotes resistance to radiation by repressing cytochrome c extrusion [51].
4. RT-Enhanced Tumor Metastasis
Tumor metastasis is the primary cause of failure in conventional cancer therapy [39]. Currently, the biological responses of normal tissues to RT-induced damage and the influence of them on metastatic proficiency are not fully understood. A recent study indicates that radiation exposure in healthy lung tissue induces a hospitable environment for metastatic growth. Mechanistically, RT induces neutrophil accumulation and activation in healthy lung tissue, leading to a range of tissue perturbations, including Notch activation [52]. Additionally, the epithelial–mesenchymal transition (EMT) is a biological process in which epithelial cells acquire mesenchymal characteristics, which makes them more invasive and metastatic. In cancer, this program is hijacked to endow cancer cells with tumor-initiating and metastatic potential [53][54]. For instance, RT can promote the expression of ADAM metallopeptidase domain 10 (ADAM10) in pancreatic cancer cells, leading to the cleavage of ephrinB2, which is expressed in stromal fibroblasts. Subsequently, the extracellular domain of ephrinB2 interacts with its receptor and drives EMT and invasion [55]. It has also been reported that RT can promote the stability of cell division cycle 6 (CDC6) protein, thereby promoting cell invasion and migration [56]. Moreover, the epithelial cell adhesion molecule (EpCAM) is associated with a hybrid epithelial–mesenchymal phenotype in breast cancer [57].
This entry is adapted from the peer-reviewed paper 10.3390/biom12091167
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Harrington, K.J.; Billingham, L.J.; Brunner, T.B.; Burnet, N.G.; Chan, C.S.; Hoskin, P.; Mackay, R.I.; Maughan, T.S.; Macdougall, J.; McKenna, W.G.; et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer 2011, 105, 628–639.
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85.
- Chandra, R.A.; Keane, F.K.; Voncken, F.E.M.; Thomas, C.R. Contemporary radiotherapy: Present and future. Lancet 2021, 398, 171–184.
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540.
- Kocakavuk, E.; Anderson, K.J.; Varn, F.S.; Johnson, K.C.; Amin, S.B.; Sulman, E.P.; Lolkema, M.P.; Barthel, F.P.; Verhaak, R. Radiotherapy is associated with a deletion signature that contributes to poor outcomes in patients with cancer. Nat. Genet. 2021, 53, 1088–1096.
- Kim, B.M.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.H.; Lee, Y.H.; Lee, T.H.; Chang, K.T.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913.
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102.
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162.
- Lei, G.; Zhang, Y.; Hong, T.; Zhang, X.; Liu, X.; Mao, C.; Yan, Y.; Koppula, P.; Cheng, W.; Sood, A.K.; et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene 2021, 40, 3533–3547.
- Yang, Y.; Wu, M.; Cao, D.; Yang, C.; Jin, J.; Wu, L.; Hong, X.; Li, W.; Lu, L.; Li, J.; et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci. Adv. 2021, 7, eabf6290.
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217.
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425.
- Zhang, J.; Wu, Q.; Zhu, L.; Xie, S.; Tu, L.; Yang, Y.; Wu, K.; Zhao, Y.; Wang, Y.; Xu, Y.; et al. SERPINE2/PN-1 regulates the DNA damage response and radioresistance by activating ATM in lung cancer. Cancer Lett. 2022, 524, 268–283.
- Zhang, P.; Wei, Y.; Wang, L.; Debeb, B.G.; Yuan, Y.; Zhang, J.; Yuan, J.; Wang, M.; Chen, D.; Sun, Y.; et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat. Cell Biol. 2014, 16, 864–875.
- Yun, M.; Yingzi, L.; Jie, G.; Guanxin, L.; Zimei, Z.; Zhen, C.; Zhi, L.; Yingjie, N.; Lunquan, S.; Tao, C.; et al. PPDPF Promotes the Progression and acts as an Antiapoptotic Protein in Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2022, 18, 214–228.
- Ni, M.; Li, J.; Zhao, H.; Xu, F.; Cheng, J.; Yu, M.; Ke, G.; Wu, X. BRD4 inhibition sensitizes cervical cancer to radiotherapy by attenuating DNA repair. Oncogene 2021, 40, 2711–2724.
- Chen, Y.; Jiang, T.; Zhang, H.; Gou, X.; Han, C.; Wang, J.; Chen, A.T.; Ma, J.; Liu, J.; Chen, Z.; et al. LRRC31 inhibits DNA repair and sensitizes breast cancer brain metastasis to radiation therapy. Nat. Cell Biol. 2020, 22, 1276–1285.
- Wang, J.; Hu, T.; Wang, Q.; Chen, R.; Xie, Y.; Chang, H.; Cheng, J. Repression of the AURKA-CXCL5 axis induces autophagic cell death and promotes radiosensitivity in non-small-cell lung cancer. Cancer Lett. 2021, 509, 89–104.
- Huang, Y.; Yang, X.; Lu, Y.; Zhao, Y.; Meng, R.; Zhang, S.; Dong, X.; Xu, S.; Wu, G. UBE2O targets Mxi1 for ubiquitination and degradation to promote lung cancer progression and radioresistance. Cell Death Differ. 2021, 28, 671–684.
- Yao, G.; Tang, J.; Yang, X.; Zhao, Y.; Zhou, R.; Meng, R.; Zhang, S.; Dong, X.; Zhang, T.; Yang, K.; et al. Cyclin K interacts with beta-catenin to induce Cyclin D1 expression and facilitates tumorigenesis and radioresistance in lung cancer. Theranostics 2020, 10, 11144–11158.
- Shimura, T.; Kakuda, S.; Ochiai, Y.; Nakagawa, H.; Kuwahara, Y.; Takai, Y.; Kobayashi, J.; Komatsu, K.; Fukumoto, M. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene 2010, 29, 4826–4837.
- Sun, J.; Zhu, Z.; Li, W.; Shen, M.; Cao, C.; Sun, Q.; Guo, Z.; Liu, L.; Wu, D. UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation. J. Exp. Clin. Cancer Res. 2020, 39, 222.
- Choi, S.H.; Yang, H.; Lee, S.H.; Ki, J.H.; Nam, D.H.; Yoo, H.Y. TopBP1 and Claspin contribute to the radioresistance of lung cancer brain metastases. Mol. Cancer 2014, 13, 211.
- Advani, S.J.; Camargo, M.F.; Seguin, L.; Mielgo, A.; Anand, S.; Hicks, A.M.; Aguilera, J.; Franovic, A.; Weis, S.M.; Cheresh, D.A. Kinase-independent role for CRAF-driving tumour radioresistance via CHK2. Nat. Commun. 2015, 6, 8154.
- Huang, Y.; Tian, Y.; Zhang, W.; Liu, R.; Zhang, W. Rab12 Promotes Radioresistance of HPV-Positive Cervical Cancer Cells by Increasing G2/M Arrest. Front. Oncol. 2021, 11, 586771.
- Larsen, B.D.; Benada, J.; Yung, P.Y.K.; Ryan, A.V.B.; George, P.; Vaclav, U.; Ahlskog, J.K.; Kuo, T.T.; Janscak, P.; Megeney, L.A.; et al. Cancer cells use self-inflicted DNA breaks to evade growth limits imposed by genotoxic stress. Science 2022, 376, 476–483.
- Clarke, M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 380, 2237–2245.
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232.
- Bayik, D.; Lathia, J.D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 2021, 21, 526–536.
- Osuka, S.; Zhu, D.; Zhang, Z.; Li, C.; Stackhouse, C.T.; Sampetrean, O.; Olson, J.J.; Gillespie, G.Y.; Saya, H.; Willey, C.D.; et al. N-cadherin upregulation mediates adaptive radioresistance in glioblastoma. J. Clin. Investig. 2021, 131, e136098.
- Li, M.Y.; Fan, L.N.; Han, D.H.; Yu, Z.; Ma, J.; Liu, Y.X.; Li, P.F.; Zhao, D.H.; Chai, J.; Jiang, L.; et al. Ribosomal S6 protein kinase 4 promotes radioresistance in esophageal squamous cell carcinoma. J. Clin. Investig. 2020, 130, 4301–4319.
- Park, S.Y.; Lee, C.J.; Choi, J.H.; Kim, J.H.; Kim, J.W.; Kim, J.Y.; Nam, J.S. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J. Exp. Clin. Cancer Res. 2019, 38, 399.
- Monteiro, C.; Miarka, L.; Perea-Garcia, M.; Priego, N.; Garcia-Gomez, P.; Alvaro-Espinosa, L.; de Pablos-Aragoneses, A.; Yebra, N.; Retana, D.; Baena, P.; et al. Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nat. Med. 2022, 28, 752–765.
- Yang, G.; Lu, C.; Mei, Z.; Sun, X.; Han, J.; Qian, J.; Liang, Y.; Pan, Z.; Kong, D.; Xu, S.; et al. Association of Cancer Stem Cell Radio-Resistance Under Ultra-High Dose Rate FLASH Irradiation with Lysosome-Mediated Autophagy. Front. Cell Dev. Biol. 2021, 9, 672693.
- Bai, X.; Ni, J.; Beretov, J.; Wang, S.; Dong, X.; Graham, P.; Li, Y. THOC2 and THOC5 Regulate Stemness and Radioresistance in Triple-Negative Breast Cancer. Adv. Sci. 2021, 8, e2102658.
- Bridges, A.E.; Ramachandran, S.; Pathania, R.; Parwal, U.; Lester, A.; Rajpurohit, P.; Morera, D.S.; Patel, N.; Singh, N.; Korkaya, H.; et al. RAD51AP1 Deficiency Reduces Tumor Growth by Targeting Stem Cell Self-Renewal. Cancer Res. 2020, 80, 3855–3866.
- Sun, X.; He, Z.; Guo, L.; Wang, C.; Lin, C.; Ye, L.; Wang, X.; Li, Y.; Yang, M.; Liu, S.; et al. ALG3 contributes to stemness and radioresistance through regulating glycosylation of TGF-beta receptor II in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 149.
- Liang, Y.Y.; Niu, F.Y.; Xu, A.A.; Jiang, L.L.; Liu, C.S.; Liang, H.P.; Huang, Y.F.; Shao, X.F.; Mo, Z.W.; Yuan, Y.W. Increased MCL-1 synthesis promotes irradiation-induced nasopharyngeal carcinoma radioresistance via regulation of the ROS/AKT loop. Cell Death Dis. 2022, 13, 131.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022, 12, 31–46.
- Fu, S.; Li, Z.; Xiao, L.; Hu, W.; Zhang, L.; Xie, B.; Zhou, Q.; He, J.; Qiu, Y.; Wen, M.; et al. Glutamine Synthetase Promotes Radiation Resistance via Facilitating Nucleotide Metabolism and Subsequent DNA Damage Repair. Cell Rep. 2019, 28, 1136–1143.e4.
- Binkley, M.S.; Jeon, Y.J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov. 2020, 10, 1826–1841.
- Zhou, W.; Yao, Y.; Scott, A.J.; Wilder-Romans, K.; Dresser, J.J.; Werner, C.K.; Sun, H.; Pratt, D.; Sajjakulnukit, P.; Zhao, S.G.; et al. Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat. Commun. 2020, 11, 3811.
- Wahl, D.R.; Dresser, J.; Wilder-Romans, K.; Parsels, J.D.; Zhao, S.G.; Davis, M.; Zhao, L.; Kachman, M.; Wernisch, S.; Burant, C.F.; et al. Glioblastoma Therapy Can Be Augmented by Targeting IDH1-Mediated NADPH Biosynthesis. Cancer Res. 2017, 77, 960–970.
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, E8247–E8256.
- Sharanek, A.; Burban, A.; Laaper, M.; Heckel, E.; Joyal, J.S.; Soleimani, V.D.; Jahani-Asl, A. OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation. Nat. Commun. 2020, 11, 4116.
- Jiang, N.; Xie, B.; Xiao, W.; Fan, M.; Xu, S.; Duan, Y.; Hamsafar, Y.; Evans, A.C.; Huang, J.; Zhou, W.; et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat. Commun. 2022, 13, 1511.
- Tan, Z.; Xiao, L.; Tang, M.; Bai, F.; Li, J.; Li, L.; Shi, F.; Li, N.; Li, Y.; Du, Q.; et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics 2018, 8, 2329–2347.
- Han, S.; Wei, R.; Zhang, X.; Jiang, N.; Fan, M.; Huang, J.H.; Xie, B.; Zhang, L.; Miao, W.; Butler, A.C.; et al. CPT1A/2-Mediated FAO Enhancement-A Metabolic Target in Radioresistant Breast Cancer. Front. Oncol. 2019, 9, 1201.
- Tirinato, L.; Marafioti, M.G.; Pagliari, F.; Jansen, J.; Aversa, I.; Hanley, R.; Nisticò, C.; Garcia-Calderón, D.; Genard, G.; Guerreiro, J.F.; et al. Lipid droplets and ferritin heavy chain: A devilish liaison in human cancer cell radioresistance. elife 2021, 10, e72943.
- Fang, Y.; Zhan, Y.; Xie, Y.; Du, S.; Chen, Y.; Zeng, Z.; Zhang, Y.; Chen, K.; Wang, Y.; Liang, L.; et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology 2021, 75, 1386–1401.
- Nolan, E.; Bridgeman, V.L.; Ombrato, L.; Karoutas, A.; Rabas, N.; Sewnath, C.; Vasquez, M.; Rodrigues, F.S.; Horswell, S.; Faull, P.; et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat. Cancer 2022, 3, 173–187.
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647.
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84.
- Mueller, A.C.; Piper, M.; Goodspeed, A.; Bhuvane, S.; Williams, J.S.; Bhatia, S.; Phan, A.V.; Van Court, B.; Zolman, K.L.; Peña, B.; et al. Induction of ADAM10 by Radiation Therapy Drives Fibrosis, Resistance, and Epithelial-to-Mesenchyal Transition in Pancreatic Cancer. Cancer Res. 2021, 81, 3255–3269.
- Yu, X.; Liu, Y.; Yin, L.; Peng, Y.; Peng, Y.; Gao, Y.; Yuan, B.; Zhu, Q.; Cao, T.; Xie, B.; et al. Radiation-promoted CDC6 protein stability contributes to radioresistance by regulating senescence and epithelial to mesenchymal transition. Oncogene 2019, 38, 549–563.
- Mal, A.; Bukhari, A.B.; Singh, R.K.; Kapoor, A.; Barai, A.; Deshpande, I.; Wadasadawala, T.; Ray, P.; Sen, S.; De, A. EpCAM-Mediated Cellular Plasticity Promotes Radiation Resistance and Metastasis in Breast Cancer. Front. Cell Dev. Biol. 2020, 8, 597673.
This entry is offline, you can click here to edit this entry!
