The control of leishmaniases, a complex parasitic disease caused by the protozoan parasite Leishmania, requires continuous innovation at the therapeutic and vaccine levels. Thus, the classical drugs are toxic and generate drug resistance. These limitations are also the consequence of a non adapted biodistribution of the active principles. Chitosan is a biocompatible polymer administrable via different routes and possessing numerous qualities to be used in the antileishmanial strategies.
- chitosan
- drug carriers, leishmaniasis
- chemotherapy
- vaccine
1. Introduction
Leishmaniases are neglected tropical and sub-tropical diseases with an estimated 0.7-1 million new cases per year in nearly 100 endemic countries, caused by Leishmania spp, a protozoan parasite transmitted by the female Phlebotomine sandfly [1][2]. About twenty Leishmaniaspecies are able to infect humans and two main clinical manifestations are usually described : visceral leishmaniasis (VL) that is fatal in the absence of treatment and which also affects dogs, and cutaneous leishmaniasis (CL), which is self-curing but leads to disfigurement and stigmatisation. There are other clinical manifestations of CL including mucocutanous and diffuse forms. Whereas some vaccines exist for dogs, with uncomplete efficacy, none are marketed for human use. Chemotherapy is presently the single approach to manage these diseases, which in combination with a more intense vector control[1].
2. Mechanism of Action of Chitosan
First data on the mechanism of action of chitosan revealed an optimal in vitro intrinsic activity at acidic pH, high-molecular-weight chitosan being the most efficient form, with an uptake by pinocytosis and an accumulation in the parasitophorous vacuole of Leishmania-infected macrophages. In addition, the immunomodulatory effect of chitosan is an added-value both for the treatment of leishmaniasis and the development of innovative vaccines. The advances in chitosan chemistry allows pharmacomodulation on amine groups opening various opportunities for new polymers of different size, and physico-chemical properties adapted to the chosen routes of administration. Different formulations have been studied in experimental leishmaniasis models to cure visceral and cutaneous leishmaniasis, and chitosan can act as a booster through drug combinations with classical drugs, such as amphotericin B. The various architectural possibilities given by chitosan chemistry and pharmaceutical technology pave the way for promising further developments.
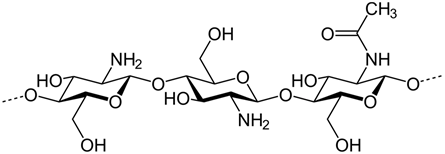
The chemical structure of chitosan is presented in Figure 1.
Figure 1. Chemical structure of chitosan polymer.
3. Future Perspectives
The first advantages of chitosan are its ease of production at low cost and its biodegradability. This review has highlighted interesting data, paving the way to further investigations on leishmaniasis both in therapeutic and vaccine research. However, despite the number of data collected, it was not possible to highlight the most relevant physico-chemical characteristics of the formulations responsible for the best in vitro selectivity index and in vivo activity, as many parameters, i.e., the nature of the polymer, the particle size, the zeta potential, the loaded drugs, and the different strains and protocols used for their biological evaluation are intimately entangled. Further studies focused only on AmB, for example, would be useful for a strict comparison between the most promising formulations by using the same in vitro and in vivo models of experimental leishmaniasis.
The second advantage of chitosan is its intrinsic antileishmanial activity. Thus, when combined with another drug, it acts as a booster and, to some extent, it can also be considered as an active principle for drug combination. Few studies have focused on the mechanism of action of chitosan on Leishmania. It is known that Leishmania sp. expresses chitinase activity thought to be important in parasite-sandfly interactions and transmission of the parasite to the vertebrate host [3]. Even if chitinases have the unique ability to hydrolyse GlcNAc-GlcNAc bonds, making these enzymes capable of hydrolysing chitin, they are able to hydrolyse, to some extent, partially acetylated chitosan as well [4]. Thus, the importance of leishmanial chitinases in the mechanism of action of chitosan is worthy of further consideration if GlcNAc oligomers exhibit intrinsic antileishmanial activity.
Significative efforts on chitosan research have been carried out for a decade in antileishmanial chemotherapy by association with drugs, and mainly AmB, as this drug is a star in antileishmanial therapy. However, improvements in activity are required via different strategies including various chitosan formulations. Thus, several parameters such as the degree of deacetylation, the particle size, and the administration route gave various possibilities and promising perspectives of chitosan for therapies and vaccines.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25184123
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970.
- Valero, N.N.H.; Uriarte, M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: A systematic review. Parasitol. Res. 2020, 119, 365–384.
- Rogers, M.E.; Hajmová, M.; Joshi, M.B.; Sadlova, J.; Dwyer, D.M.; Volf, P.; Bates, P.A. Leishmania chitinase facilitates colonization of sandfly vectors and enhances transmission to mice. Cell Microbiol. 2008, 10, 1363–1372.
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczesna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan and chito-oligosaccarides. Front. Bioeng. Biotechnol. 2019, 7, 243.