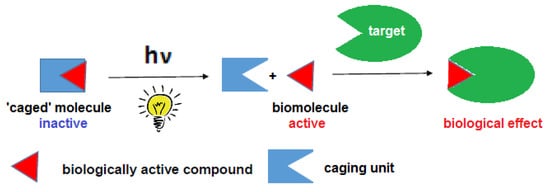
Photoremovable protecting groups (PPGs) (also often called photocages in the literature) are used for temporary inactivation of biologically active substrates. By photoirradiation the PPG could be cleaved off and the biological activity could be restored on-demand, with a high spatiotemporal precision. The on-site liberation of the biologically active substrate could be exploited for studying dynamic biological processes or for designing targeted pharmacological interventions in vitro or in vivo. Several chemical scaffolds have been described and tested as PPGs, operating at different wavelengths. The scope of potential substrates is very broad, spanning from small molecules to proteins. In a wider context, PPGs could be used for the design of various light-responsive materials as well, for diverse applications.
- photoremovable protecting groups
- photocages
- photoactivation
- uncaging
- two-photon irradiation
- drug delivery
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia2030082
References
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117.
- Monteiro, D.C.F.; Amoah, E.; Rogers, C.; Pearson, A.R. Using photocaging for fast time-resolved structural biology studies. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 1218–1232.
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Methods 2007, 4, 619–628.
- Klán, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191.
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178.
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747.
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447.
- Korzycka, K.A.; Bennett, P.M.; Cueto-Diaz, E.J.; Wicks, G.; Drobizhev, M.; Blanchard-Desce, M.; Rebane, A.; Anderson, H.L. Two-photon sensitive protecting groups operating via intramolecular electron transfer: Uncaging of GABA and tryptophan. Chem. Sci. 2015, 6, 2419–2426.
- Piant, S.; Bolze, F.; Specht, A. Two-photon uncaging, from neuroscience to materials. Opt. Mater. Express 2016, 6, 1679.
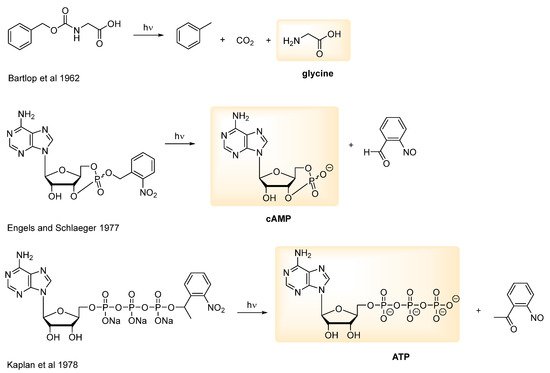
- Engels, J.; Schlaeger, E.J. Synthesis, structure, and reactivity of adenosine cyclic 3′,5′-phosphate-benzyltriesters. J. Med. Chem. 1977, 20, 907–911.
- Kaplan, J.H.; Forbush, I.B.; Hoffman, J.F. Rapid photolytic release of adenosine 5′-triphosphate from a protected analog: Utilization by the sodium:potassium pump of human red blood cell ghosts. Biochemistry 1978, 17, 1929–1935.
- Barltrop, J.; Schofield, P. Photosensitive Protecting Groups. Tetrahedron Lett. 1962, 3, 697–699.
- Hurevich, M.; Samarasimhareddy, M.; Alshanski, I.; Mervinetsky, E. Photodeprotection of up to Eight Photolabile Protecting Groups from a Single Glycan. Synlett 2018, 29, 880–884.
- Kessler, M.; Glatthar, R.; Giese, B.; Bochet, C.G. Sequentially Photocleavable Protecting Groups in Solid-Phase Synthesis. Org. Lett. 2003, 5, 1179–1181.
