Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Today, inactivity and high-calorie diets contribute to the development of obesity and premature aging. In addition, the population of elderly people is growing due to improvements in healthcare management. Obesity and aging are together key risk factors for non-communicable diseases associated with several co-morbidities and increased mortality, with a major impact on skeletal muscle defect and/or poor muscle mass quality.
- grape polyphenols
- resveratrol
- sarcopenia
- skeletal muscle
1. Obesity and Aging: Two Major Healthcare Challenges to Solve
The population of elderly people is expanding worldwide with the older adults aged between 65–80 years being the fastest-growing portion, thanks to improvements in healthcare management which allows for increasing life expectancy [1]. Associated with this increase in lifespan, a global obesity epidemic is spreading due to life changes such as inactivity and high-calorie diets, favoring the growth of non-communicable diseases. Obesity and aging are together key risk factors for the development and progression of several chronic/non-communicable diseases (metabolic syndrome, Insulin Resistance (IR), Type 2 Diabetes (T2D) [2][3][4][5][6][7], sarcopenia [8][9], and frailty [10]. The World Health Organization (WHO) defines metabolic syndrome as a pathologic condition characterized by obesity (Body Mass Index (BMI) ≥30 kg/m2), IR, hypertension, and hyperlipidemia [11]). T2D is IR associated with decreased insulin secretion by the pancreas. [12]. Frailty is a clinical syndrome in elderly people comprising an increased risk for poor health outcomes, falls, incident disability, hospitalization, and mortality [13].
1.1. Skeletal Muscle Alterations Are Central
Although non-communicable diseases associated with aging and obesity have different etiologies, development, and progression, they are all associated with skeletal muscle defect and/or poor muscle mass quality. Sarcopenia, defined as a loss of skeletal muscle mass and function [8], is frequently associated with aging and with a loss of independence, disability, frailty, and compromised quality of life, and, therefore, represents a high risk for morbidity and mortality [9]. Obese patients could also develop sarcopenia [7] and with the progression of obesity with aging [14], a growing number of obese sarcopenic patients is expected. Then, the management of skeletal muscle alteration during obesity and aging is mandatory.
Skeletal muscles are among the major tissues of the body, accounting for 40% of the total body weight and containing 50–75% of all body proteins. Moreover, skeletal muscles are responsible for more than 80% of glucose uptake after insulin stimulation, highlighting their central role in metabolism regulation. They ensure three main functions: posture and locomotion, thermoregulation, storage, and utilization of nutrients. Thus, they play a vital role in all activities [15]. Growing evidence points to the central role of skeletal muscle in the systemic regulation of age-related diseases [16]. Indeed, functional and metabolic muscle alterations, as well as skeletal muscle mass decreasing, are all associated with the human mortality rate [17][18]. Then, impairment of skeletal muscle mass and/or function could lead to major pathologies, such as IR, T2D, and to weakness and disability which considerably decrease quality of life and are associated with a higher risk for morbidity and mortality [5][7][19][20].
1.2. Muscle Alterations
1.2.1. Muscle Alterations in Obesity
Obesity exerts multiple effects on skeletal muscle metabolism. In obese grade I insulin-resistant women, only skeletal muscle insulin-signaling alteration was found with no variation in subcutaneous adipose tissue [21]. Moreover, the correlation of muscle alteration with glucose infusion rate during the hyperinsulinemic-euglycemic clamp underlines the major role of skeletal muscle in IR development [21]. Obesity is accompanied by increased ectopically lipid deposition in non-adipose tissues including skeletal muscle [22][23]. This lipid overload affects several cell signaling pathways and is associated with metabolic effects on mitochondrial function, insulin response, and energetic metabolism [24][25] (Figure 1). After their cell translocation by fatty acid translocase (FAT/CD36), a receptor and transporter for free fatty acid (FFA), lipids are stored as intramuscular triacylglycerols (TAG) in lipid droplets (IMTG). Myotubes from obese people present increased FFA uptake and esterification into complex lipids with overexpression of FAT/CD36 [23][26][27]. TAG turnover is also highly altered due to decreased lipolysis as lower hormone-sensitive lipase (HSL) and higher adipose triglyceride lipase (ATGL) protein levels are detected in the skeletal muscle of obese subjects [28]. Increased IMTG is also associated with higher levels of lipotoxic intermediates such as diacylglycerols (DAG) and ceramides. DAG might inhibit insulin signaling via the activation of the protein kinase C (PKC), which, in turn, decreases the activities of the phosphoinositide 3-kinase (PI3K) and of the insulin receptor substrate-1 (IRS1) in the insulin signaling pathway [29]. Ceramides have several functions in the alteration of skeletal muscle metabolism. They have been described as inhibitors of Akt phosphorylation via the activation of protein phosphatase 2A (PP2A) or, on the opposite, as inductors of Akt phosphorylation on an inhibitory residue via the activation of the protein kinase Cζ (PKCζ). Ceramides act also at the mitochondrial level by decreasing mitochondrial respiration, inhibiting oxidative phosphorylation, and promoting mitochondrial fragmentation. These activities lead to an induction of reactive oxidative species (ROSP) [30]. Decreased mitochondrial content in muscle of obese patients has also been attributed to impairment of mitochondrial biogenesis, decreasing the ability to oxidize lipids [31]. More recently, it was shown that mitochondrial lipid oxidation was impaired due to a decreased activity of the mitochondrial protein carnitine palmitoyltransferase 1 (CPT1). CPT1 is involved in the transport of long-chain fatty acids into the mitochondria. Then decreased CPT1 activity results in decreased β oxidation [32]. Circulating FFA could also induce a chronic low-grade inflammation through activation of toll-like receptor 4 (TLR4) and nuclear factor-kappa B (NFκB), resulting in the release of several pro-inflammatory cytokines as interleukines (IL) 6, 8, and 15 and tumor necrosis factor (TNF) α. These cytokines, as secreted by muscle, are also known as myokines. They could exert an autocrine or paracrine effect. However, it must be kept in mind that there is not a consensus on their secretion and their role in skeletal muscle inflammation and skeletal muscle metabolism dysregulation during obesity [21][33] Importantly, lipids overload also affects muscle maintenance and regeneration [34]. FFA accumulation decreases AMPKα activity. AMPKα promoted myogenesis by regulating the expression of miR-206 and miR-206’s target cyclin D1, which allows the regulation of the cell cycle and cell proliferation of muscular stem cells (satellite cells) during the skeletal muscle regeneration process [35]. By affecting general skeletal muscle function, mass, and quality, obesity reduces mobility [36] modifies lipid and carbohydrate metabolism and increases the risk of several comorbidities [37]. In fact, obesity accelerates the aging process and decreases life expectancy. Forty-year-old obese non-smoker females lost 7.1 years and forty-year-old obese non-smoker males lost 5.8 years of life expectancy [38].
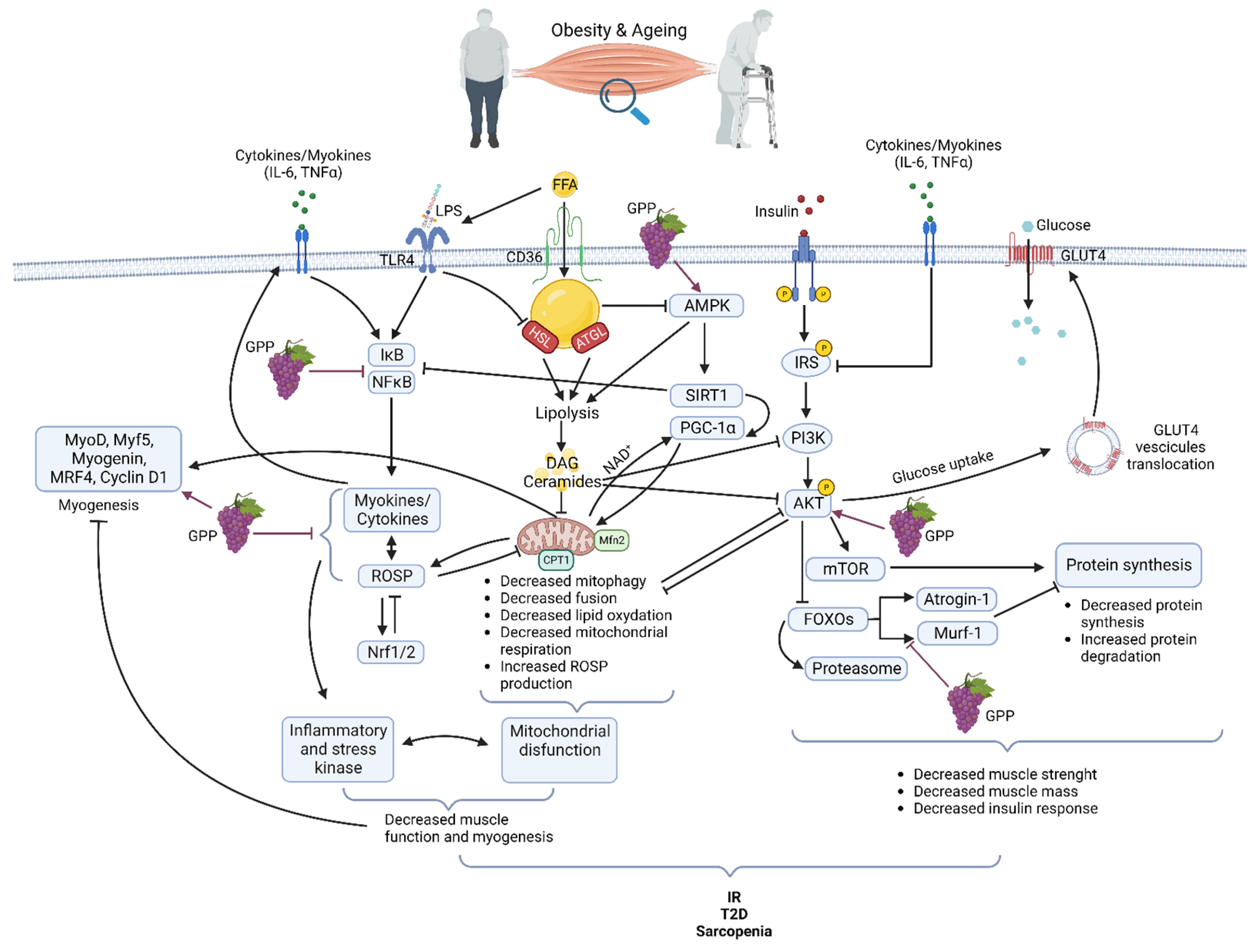
Figure 1. Main pathways involved in muscle dysfunction during obesity and aging and their modifications by grape polyphenols (GPP) (Created with BioRender.com).
1.2.2. Muscle Alterations in Aging
The gradual decline of all physiological functions of all organs including skeletal muscle characterizes normal aging. During aging, due to an alteration of muscle quality, the loss of muscle strength precedes the loss of muscle mass [39][40]. However, muscle mass is lost as early as the fifth decade with acceleration from the age of 70 and sarcopenia is a hallmark of the aging process. Like in obesity, the reduction of muscle quality and mass with aging is the consequence of the interplay of a multitude of mechanisms [41] including damage caused by ROSP [42] inflammation [43], lipid infiltration [44], proteostasis imbalance [45] and mitochondrial impairment [46]. In aging like in obesity, alteration in mitochondria content, fusion, fission, and function are associated with muscle loss and muscle lipid accumulation [46] (Figure 1). Progressive mitochondrial dysfunction and ROSP accumulation are central to the aging process [47]. Aged skeletal muscle accumulates dysfunctional mitochondria due to a defect in mitophagy, mitochondrial biogenesis, and dynamics. Reduced expression levels of genes such as nuclear respiratory factor 1/2 (Nrf1/2), AMP-activated protein kinase (AMPK), peroxisome proliferative activated receptor gamma, coactivator 1 alpha (PGC-1α), mitofusin 1 and 2 (Mfn1/2) causes a reduction of mitochondrial number, mitochondrial content, mtDNA copy number, and impairment in mitochondria morphology in the skeletal muscle. Throughout life, the accumulation of dysfunctional mitochondria producing high ROSP levels contributes to the establishment of oxidative stress and increased risk of IR and T2D. Moreover, these defective mitochondria are unable to sustain enough energy in the cells, which results in progressive functional decline and cell death. [48][49][50]. Enhancement of ectopic fat deposit during aging is also accompanied by a resulting heightened production of pro-inflammatory cytokines (IL-6, TNFα) associated with lipotoxicity and leading to an increased risk of IR and T2D [51]. Protein quality control pathways, autophagy, and proteasome activity decrease participate also in the skeletal muscle dysfunctions and the decreased muscle mass observed during aging [45][52]. Maintaining muscle mass is a balance between protein synthesis and protein degradation systems. Aged skeletal muscle shows a marked defect in the contraction-induced activation of the protein synthesis pathway phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR). Concerning the proteasome pathway and the muscle-specific ubiquitin ligase muscle RING-finger protein-1 (MuRF1) and atrogin-1 mRNA, levels in aged muscle are increased or unchanged [53][54].
The aging process presents several common mechanisms for obesity. On the other side, obesity in elderly people accelerates the aging process. Obesity and aging both deregulate cell metabolism and create a vicious circle that precipitates the aging process and the development of associated comorbidities [41][55]. Several mechanisms operating at different levels of muscle physiology are implicated in the muscle defects observed in obesity and aging. However, oxidative stress and inflammation are central components at the onset of muscle defect regardless of its etiology [6][19][56][57][58].
2. Oxidative Stress and Inflammation: Two Essential Harms
Oxidative stress occurs when there is an imbalance between the production of ROSP in the cells and tissues and the antioxidant systems, which are responsible for their neutralization and removal [59]. Reactive oxidative species include derivatives of oxygen (Reactive Oxygen Species, ROS), nitrogen (Reactive Nitrogen Species, RNS), and sulfur (Reactive Sulfur Species, RSS), capable of oxidizing different substrates. The antioxidant defense system involves non-enzymatic scavengers provided by food as vitamins (trans retinol 2, vitamin A; ascorbic acid, vitamin C; α-tocopherol, vitamin E), carotenoids, polyphenols, and endogenous antioxidant enzymes superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione transferase (GST) (see [60][61] for complete reviews).
If an excess of ROSP can be extremely deleterious, their regular generation is necessary for the physiological maintenance of all the tissues of human body and among them, the skeletal muscle [62]. In fact, via oxidation of redox-sensitive protein-cysteine, ROSP act as second messengers. They are important signaling molecules regulating metabolism, cell growth and differentiation, cell repair, immunity, and so on [59][63]. ROSP production is thus beneficial and essential to cell and tissue function. As oxidative stress, inflammation is also essential for normal organ function. Inflammation is a protective biological response aimed at identifying and eliminating a threat. Inflammation could be triggered by infection or not and participates in the activation of the immune system. It is the first line of defense against pathogens, but it also allows for repairing cell damage and tissue injury [64][65]. ROSP production and inflammation have dual roles. They are activated during several physiological responses, and they play an essential role in cellular signaling and regulatory pathways. However, they must be tightly regulated to avoid the development of oxidative stress, tissue injury, and chronic inflammation, which are detrimental to normal cells and tissues. In fact, when ROSP are produced in excess or inadequately they can cause irreversible damage to cells by oxidizing plasma membranes, DNA, proteins, and lipids [66]. In the same way, uncontrolled inflammation could lead to chronic inflammation and inflammatory diseases that could affect absolutely all the organs as skeletal muscle [64][67]. Interestingly, ROSP and inflammation could regulate each other in a two-way reciprocal direction [51]. Both ROSP production and inflammation participate together in skeletal muscle tissue repair [52]. However, during obesity and aging, ROSP production and inflammation are increased while antioxidant systems and anti-inflammatory pathways decreased in skeletal muscle [21][52][53]. In obesity and aging, high plasma levels of free fatty acids (FFA) and increased concentrations of lipopolysaccharide (LPS) from gut microbiota (due to increased permeability of gut), bind to Toll-like Receptor 4 (TLR4) [68][69]. TLR4 is an innate immunity receptor present in the skeletal muscle that can activate NFκB and inflammation via the MyD88 pathway. Moreover, the E3 ubiquitin ligase RNF41 has been found to participate in the TLR4 inflammation pathway in the muscle of insulin-resistant grade I obese women [56]. An excess of FFA is also responsible for deleterious effects on mitochondria such as uncoupling of oxidative phosphorylation, energy failure, decreased clearance, decreased fission, and release of ROSP [70]. Mitochondrial dysfunction and inflammation/oxidative stress together are responsible for a decrease in myogenesis and muscle function [71]. In skeletal muscle, elevated ROSP levels concurrently inhibit anabolic pathways as PI3K/Akt/mTOR [72], contributing to muscle mass loss and atrophy [73][74], and activating several mechanisms of the catabolic pathways (Figure 1). Under physiological conditions, Akt phosphorylates and inhibits the Forkhead box O transcription factors (FOXOs), thus inhibiting the muscle-specific ubiquitin ligase MuRF1 and atrogin-1, of the ubiquitin-proteasome system. With the inhibition of Akt, atrogin-1, MuRF-1 and proteasome are activated resulting in protein degradation [75]. Activation of the cysteine proteases, calpain, and caspase 3, which play a key role in the initial breakdown of sarcomeres during atrophic conditions, also participates in protein breakdown and muscle atrophy. The expression of inflammatory myokines such as tumor necrosis factor-alpha (TNFα) and interleukin 6 (IL6) is induced due to an increase in the activity of the transcription factor nuclear factor kappa B (NFκB) (Figure 1). NFκB activity can be increased by ROSP but also by several other stimuli such as free fatty acids (FFA), advanced glycation products, and inflammatory cytokines induced by oxidative stress [19]. On the other way, high levels of ROSP activate the nuclear factor, erythroid 2-like 2 (Nrf2) pathway leading to increased transcription of genes coding for antioxidant proteins, and consequently inducing the antioxidant ROSP-fighting effects [76].
3. Grape Polyphenols: An Effective Tool
Adapted diet and physical activity are known for several years as central countermeasures to avoid the deleterious physiological effects of obesity and aging on tissue homeostasis and to promote a healthy life. Treatment of obesity is difficult, and initially based on lifestyle change, diet recommendations, and increased physical activity [77] but, whereas it is actually effective, it is associated with very low compliance. Aging is inevitable but it is desirable to age in a healthy way, therefore the purpose of human society is now to increase the rate of healthy aging to avoid harmful consequences on skeletal muscle mass and function and to limit frailty and dependence. As stated above, oxidative stress and chronic inflammation are core mechanisms associated with obesity and aging. Among the numerous natural chemical compounds tested for their antioxidant and anti-inflammatory properties, grape polyphenols present great interest.
Attention to the importance of dietary intake of polyphenols was ignited by the phenomenon called the ‘French Paradox’, first described by Serge Renaud from the University of Bordeaux in 1992. According to his observations, the French population when compared to other Western populations whose diet is rich in saturated fatty acids (e.g., the American population), shows a much lower incidence of coronary heart disease and associated mortality [78]. In fact, adherence to the Mediterranean diet which includes mainly plant-derived foods and red wine consumption has been associated with a lower risk of chronic diseases and mortality [79] and a lower frailty index in older adults [80]. The first explanation put forward to explain these associations was the moderate consumption of red wine. Undeniably, among fruits, grapes (but also red wine, grape seeds, and grape pomace) contain high amounts of polyphenols [81] (Table 1), although not being the richest source. This does not affect its great biological interest due to the exceptional variety of polyphenol families and molecules of known beneficial activity on human health that can all be found in it (Table 2).
Table 1. Polyphenols characterization, total polyphenol content (TPC) of major well-known vegetal sources of dietary polyphenols. TPC is expressed in mg/100 g of fresh weight (FW). Data in the table were extracted from the PhenolExplorer database [82][83][84].
| Source | Family of Polyphenol | Amount | Mean TPC (Folin Assay) |
|---|---|---|---|
| Apple | Anthocyanins | 0.93 mg/100 g FW | 200.96 mg/100 g FW |
| Dihydrochalcones | 5.38 mg/100 g FW | ||
| Flavanols | 24.12 mg/100 g FW | ||
| Flavonols | 6.86 mg/100 g FW | ||
| Phenolic acids | 19 mg/100 g FW | ||
| Artichoke, heads, raw | Flavones | 57.8 mg/100 g FW | 1142.40 mg/100 g FW |
| Phenolic acids | 202.23 mg/100 g FW | ||
| Blueberries | Flavonols | 12.23 mg/100 g FW | 151.33 mg/100 g FW |
| Phenolic acids | 162.47 mg/100 g FW | ||
| Phenolic acids | 37.06 mg/100 g FW | ||
| Other polyphenols | 0.45527 mg/100 g FW | ||
| Cocoa, powder | Flavanols | 511.62 mg/100 g FW | 5624.23 mg/100 g FW |
| Phenolic acids | 37.06 mg/100 g FW | ||
| Other polyphenols | 0.45527 mg/100 g FW | ||
| Grape | Anthocyanins | 72.1 mg/100 g FW | 184.97 mg/100 g FW |
| Flavanols | 17.11 mg/100 g FW | ||
| Flavonols | 3.08 mg/100 g FW | ||
| Phenolic acids | 1.69 mg/100 g FW | ||
| Stilbenes | 0.3362 mg/100 g FW | ||
| Green tea | Flavanols | 71.18 mg/100 g FW | 61.86 mg/100 ml |
| Flavonols | 5.29 mg/100 g FW | ||
| Phenolic acids | 12.53 mg/100 g FW | ||
| Olives, green | Flavones | 0.56 mg/100 g FW | 161.24 mg/100 g FW |
| Phenolic acids | 134.94 mg/100 g FW | ||
| Other polyphenols | 211.05 mg/100 g FW | ||
| Persil, fresh | Other polyphenols | 13.95 mg/100 g FW | 89.27 mg/100 g FW |
| Strawberries | Anthocyanins | 73.01 mg/100 g FW | 289.20 mg/100 g FW |
| Flavanols | 9.1375 mg/100 g FW | ||
| Flavonols | 2.32 mg/100 g FW | ||
| Phenolic acids | 10.74 mg/100 g FW | ||
| Stilbenes | 0.35 mg/100 g FW |
Table 2. Main effects of polyphenols present in grapes on mechanisms involved in obesity and aging.
| Family and Subfamily | Compound | Effect and Mechanism | References |
|---|---|---|---|
| Flavonoids/Flavan-3-ols | EGCG | ● Antioxidant Radical scavenging Metal ion chelation ↑ CAT, ↑ SOD1 e SOD2, ↑ GPx ● Anti-inflammatory ↓ NFĸB via ↓ Iĸβ ↓ COX-2 ↓ IRF3 via ↓ TBK1 ● Anti-diabetic ↓ insulin resistance ↑ lipid oxidation in muscle ↑ NFĸB, ↑ AMPK, ↑ MAPK ● Anti-aging/pro-apoptotic ↑ Beclin-1 and ↑ caspases |
● Fraga et al. [85] Bernatoniene et al. [86] Meng et al. [57] ● Youn et al. [87] ● Casanova et al. [88] Li et al. [89] ● Pallauf and Rimbach [90] |
| Grape seed proanthocyanidins | ● Anti-diabetic ↑ Nrf1, ↑SIRT1, and ↑PGC-1α, ↑ slow myosin heavy chain, ↑ succinic dehydrogenase and malate dehydrogenase activities, ↑ resistance to fatigue |
● Xu et al. [91] | |
| Flavonoids/Flavanols | Quercetin | ● Anti-inflammatory ↑ Nrf2/ARE pathways ↑ Antioxidant enzymes ↓ TNF-α, ↓ IL-6, ↓ IL-1β, ↓ COX-2, ↓ iNOS, ↓ NFĸB in adipocytes and macrophages |
● Costa et al. [92] ● Sato et al. [93] |
| Myricetin | ● Anti-diabetic ↑ glucose uptake, ↓ insulin resistance, ↑Akt and ↑AMPK signaling pathways |
● Pandey et al. [94] | |
| Kaempferol | ● Anti-inflammatory ↓ IL-6, IL-1β, 18 and TNF-α ↑ Nrf2 and synthesis targets Inhibition TLR4 |
● Alam et al. [95] | |
| Flavonoids/Anthocyanes | Anthocyanins | ● Anti-inflammatory ↓ COX-1 and COX-2 ↓ C-reactive protein |
● Mozos et al. [96] ● Sivamaruthi et al. [97] |
| Cyanidin-3-O-glucoside | ● Antidyslipidemic ↑ PPARs ● Anti-diabetic ↑ Insulin sensitivity → ↑ PPARs ↑ Insulin secretion → ↓ IL-1β and IL-6 ↑ TLR4/IĸBα pathway |
● Jia et al. [98] ● Geng et al. [99] |
|
| Flavonoids/Flavones | Luteolin/Apigenin | ● Anti-inflammatory ↓ NO and ↓ PGE2 |
● Tian et al. [100] |
| Apigenin | ● Anti-obesity Radical scavenger ↑ Increase muscle fibers size ↑ number and volume mitochondria ↑ SOD and GPx |
● Wang et al. [101] | |
| Flavonoids/Isoflavones | Daidzein | ● Anti-diabetic Inhibition α-amylase and α-glycosidase ↑ AMPK, ↑ GK, ↓ G6Pase, ↓ PEPCK, ↑ GLUT4, ↑ IRS1, ↑ IRS2, ↑ PPARγ ● Anti-inflammatory ↑ PPARγ, ↓ TNFα, ↓ NFĸB, ↓ IL-6, ↓ Ccl2, ↓ Cxcl2 |
● Park et al. [102] ● Das et al. [103] |
| Genistein | ● Anti-diabetic ↑ AMPK in skeletal muscle ↑ insulin sensitivity ↑ lipid oxidation |
● Guevara-Cruz et al. [104] | |
| Flavonoids/Flavanones | Naringenin | ● Anti-diabetic ↑ Insulin secretion ● Anti-inflammatory ↓ TNF-α and IL-6 ↑ SOD |
● Rehman et al. [105] |
| Hesperidin | ● Anti-diabetic ↑ IRS, ↑ Akt, and ↑ GLUT4 in muscle cells |
● Dhanya et al. [106] | |
| Stilbenes | Resveratrol | ● Anti-diabetic ↑ SIRT1 and ↑ PGC-1α ↑ mitochondrial activity (exercise mimetic effect) ↑ Akt and AMPK pathways → ↑ insulin sensitivity ● Anti-obesity ↓ fat accumulation ↑ lipolysis ● Anti-aging ↓ caspase 3 |
● Lagouge et al. [107] ● Lagouge et al. [107] Kang et al. [108] ● Huang et al. [109] ● Bai et al. [110] |
| Phenolic acids | Phenolic acids | ● Anti-diabetic ↑ GLUT2 in pancreatic β-cells ↑ PI3K/Akt and ↑ GLUT4 in adipose and muscle tissues ↓ α-glucosidase activity |
● Kumar et al. [111] ● Duboit et al. [96] |
| Gallic acid/p-coumaric acid | ● Anti-diabetic and anti-obesity ↓ TNF-α and ↓ PPAR γ in adipose tissue |
● Abdel-Moneim et al. [112] | |
| Caffeic acid phenetyl ester | ● Anti-inflammatory ↓ COX and ↓ LOX Inhibition detachment arachidonic acid. |
● Silva et al. [113] | |
| Vanillic acid | ● Anti-obesity ↓ PPAR and C/EBPα ↑ Lipid oxidation through ↑ AMPKα |
● Jung et al. [114] | |
| Syringic acid | ● Anti-diabetic ↑ PGC-1α and Nrf2 ↑ increased mitochondrial biogenesis. ↓ TNF-α, IL-1β, and IL-6 |
● Rashedinina et al. [115] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196594
References
- Zarulli, V.; Sopina, E.; Toffolutti, V.; Lenart, A. Health Care System Efficiency and Life Expectancy: A 140-Country Study. PLoS ONE 2021, 16, e0253450.
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111.
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the Epidemiology of Multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068.
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern Concepts in Cardiovascular Disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211.
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27.
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55.
- Nunan, E.; Wright, C.L.; Semola, O.A.; Subramanian, M.; Balasubramanian, P.; Lovern, P.C.; Fancher, I.S.; Butcher, J.T. Obesity as a Premature Aging Phenotype—Implications for Sarcopenic Obesity. GeroScience 2022, 44, 1393–1405.
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018, 47, 123–132.
- Tian, S.; Xu, Y. Association of Sarcopenic Obesity with the Risk of All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies: Sarcopenic Obesity and Mortality. Geriatr. Gerontol. Int. 2016, 16, 155–166.
- Afonso, C.; Sousa-Santos, A.R.; Santos, A.; Borges, N.; Padrão, P.; Moreira, P.; Amaral, T.F. Frailty Status Is Related to General and Abdominal Obesity in Older Adults. Nutr. Res. 2021, 85, 21–30.
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Models Mech. 2009, 2, 231–237.
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7.
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15.
- Santos, A.L.; Sinha, S. Obesity and Aging: Molecular Mechanisms and Therapeutic Approaches. Ageing Res. Rev. 2021, 67, 101268.
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195.
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. The Influence of Skeletal Muscle on Systemic Aging and Lifespan. Aging Cell 2013, 12, 943–949.
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as Independent Risk Factor for Mortality in Chronic Heart Failure. Lancet 1997, 349, 1050–1053.
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B359–B365.
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxidative Med. Cell. Longev. 2021, 2021, 4493817.
- Bielecka-Dabrowa, A.; Ebner, N.; Santos, M.R.; Ishida, J.; Hasenfuss, G.; Haehling, S. Cachexia, Muscle Wasting, and Frailty in Cardiovascular Disease. Eur. J. Heart Fail. 2020, 22, 2314–2326.
- Amouzou, C.; Breuker, C.; Fabre, O.; Bourret, A.; Lambert, K.; Birot, O.; Fédou, C.; Dupuy, A.-M.; Cristol, J.-P.; Sutra, T.; et al. Skeletal Muscle Insulin Resistance and Absence of Inflammation Characterize Insulin-Resistant Grade I Obese Women. PLoS ONE 2016, 11, e0154119.
- Aguer, C.; Mercier, J.; Kitzmann, M. Lipid Content and Response to Insulin Are Not Invariably Linked in Human Muscle Cells. Mol. Cell. Endocrinol. 2010, 315, 225–232.
- Aguer, C.; Foretz, M.; Lantier, L.; Hebrard, S.; Viollet, B.; Mercier, J.; Kitzmann, M. Increased FAT/CD36 Cycling and Lipid Accumulation in Myotubes Derived from Obese Type 2 Diabetic Patients. PLoS ONE 2011, 6, e28981.
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal Muscle Energy Metabolism in Obesity. Obesity 2021, 29, 1582–1595.
- Borén, J.; Taskinen, M.-R.; Olofsson, S.-O.; Levin, M. Ectopic Lipid Storage and Insulin Resistance: A Harmful Relationship. J. Intern. Med. 2013, 274, 25–40.
- Bell, J.A.; Reed, M.A.; Consitt, L.A.; Martin, O.J.; Haynie, K.R.; Hulver, M.W.; Muoio, D.M.; Dohm, G.L. Lipid Partitioning, Incomplete Fatty Acid Oxidation, and Insulin Signal Transduction in Primary Human Muscle Cells: Effects of Severe Obesity, Fatty Acid Incubation, and Fatty Acid Translocase/CD36 Overexpression. J. Clin. Endocrinol. Metab. 2010, 95, 3400–3410.
- Løvsletten, N.G.; Rustan, A.C.; Laurens, C.; Thoresen, G.H.; Moro, C.; Nikolić, N. Primary Defects in Lipid Handling and Resistance to Exercise in Myotubes from Obese Donors with and without Type 2 Diabetes. Appl. Physiol. Nutr. Metab. 2020, 45, 169–179.
- Laurens, C.; Moro, C. Intramyocellular Fat Storage in Metabolic Diseases. Horm. Mol. Biol. Clin. Investig. 2016, 26, 43–52.
- Kitessa, S.; Abeywardena, M. Lipid-Induced Insulin Resistance in Skeletal Muscle: The Chase for the Culprit Goes from Total Intramuscular Fat to Lipid Intermediates, and Finally to Species of Lipid Intermediates. Nutrients 2016, 8, 466.
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330.
- Holloway, G.P.; Thrush, A.B.; Heigenhauser, G.J.F.; Tandon, N.N.; Dyck, D.J.; Bonen, A.; Spriet, L.L. Skeletal Muscle Mitochondrial FAT/CD36 Content and Palmitate Oxidation Are Not Decreased in Obese Women. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1782–E1789.
- Schlaepfer, I.R.; Joshi, M. CPT1A-Mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046.
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54.
- Akhmedov, D.; Berdeaux, R. The Effects of Obesity on Skeletal Muscle Regeneration. Front. Physiol. 2013, 4, 371.
- Jiang, A.; Guo, H.; Zhang, L.; Jiang, X.; Zhang, X.; Wu, W.; Liu, H. Free Fatty Acid Impairs Myogenic Differentiation through the AMPKα-MicroRNA 206 Pathway. Mol. Cell. Biol. 2022, 42, e00327-21.
- Tallis, J.; James, R.S.; Seebacher, F. The Effects of Obesity on Skeletal Muscle Contractile Function. J. Exp. Biol. 2018, 221, jeb163840.
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and Mortality Associated with Obesity. Ann. Transl. Med. 2017, 5, 161.
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Mamun, A.A.; Bonneux, L.; NEDCOM, The Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Ann. Intern. Med. 2003, 138, 24.
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064.
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422.
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and Ageing: Two Sides of the Same Coin. Obes. Rev. 2020, 21, e12991.
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential Implications of Polyphenols on Aging Considering Oxidative Stress, Inflammation, Autophagy, and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193.
- Peake, J.; Gatta, P.D.; Cameron-Smith, D. Aging and Its Effects on Inflammation in Skeletal Muscle at Rest and Following Exercise-Induced Muscle Injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495.
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B.; Health ABC Study. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 324–333.
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin–Proteasome System by the FoxO Transcriptional Network during Muscle Atrophy. Nat. Commun. 2015, 6, 6670.
- Romanello, V. The Interplay between Mitochondrial Morphology and Myomitokines in Aging Sarcopenia. Int. J. Mol. Sci. 2020, 22, 91.
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and Ageing: Role in Heart, Skeletal Muscle and Adipose Tissue: Mitochondria and Ageing. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369.
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial Fission and Remodelling Contributes to Muscle Atrophy. EMBO J. 2010, 29, 1774–1785.
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The Role of Mitochondria in Aging. J. Clin. Investig. 2018, 128, 3662–3670.
- Huang, D.-D.; Fan, S.-D.; Chen, X.-Y.; Yan, X.-L.; Zhang, X.-Z.; Ma, B.-W.; Yu, D.-Y.; Xiao, W.-Y.; Zhuang, C.-L.; Yu, Z. Nrf2 Deficiency Exacerbates Frailty and Sarcopenia by Impairing Skeletal Muscle Mitochondrial Biogenesis and Dynamics in an Age-Dependent Manner. Exp. Gerontol. 2019, 119, 61–73.
- Conte, M.; Martucci, M.; Sandri, M.; Franceschi, C.; Salvioli, S. The Dual Role of the Pervasive “Fattish” Tissue Remodeling With Age. Front. Endocrinol. 2019, 10, 114.
- Kaushik, S.; Cuervo, A.M. Proteostasis and Aging. Nat. Med. 2015, 21, 1406–1415.
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Current Understanding of Sarcopenia: Possible Candidates Modulating Muscle Mass. Pflugers Arch. Eur. J. Physiol. 2015, 467, 213–229.
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and Sarcopenia. Endocrine 2013, 43, 12–21.
- Jura, M.; Kozak, L.P. Obesity and Related Consequences to Ageing. Age 2016, 38, 23.
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472.
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526.
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative Stress—Complex Pathological Issues Concerning the Hallmark of Cardiovascular and Metabolic Disorders. Biomed. Pharmacother. 2022, 152, 113238.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763.
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19.
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145.
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218.
- Cooke, J.P. Inflammation and Its Role in Regeneration and Repair: A Caution for Novel Anti-Inflammatory Therapies. Circ. Res. 2019, 124, 1166–1168.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal Muscle as Potential Central Link between Sarcopenia and Immune Senescence. EBioMedicine 2019, 49, 381–388.
- Guijarro-Muñoz, I.; Compte, M.; Álvarez-Cienfuegos, A.; Álvarez-Vallina, L.; Sanz, L. Lipopolysaccharide Activates Toll-like Receptor 4 (TLR4)-Mediated NF-ΚB Signaling Pathway and Proinflammatory Response in Human Pericytes. J. Biol. Chem. 2014, 289, 2457–2468.
- Lee, S.; Norheim, F.; Gulseth, H.L.; Langleite, T.M.; Kolnes, K.J.; Tangen, D.S.; Stadheim, H.K.; Gilfillan, G.D.; Holen, T.; Birkeland, K.I.; et al. Interaction between Plasma Fetuin-A and Free Fatty Acids Predicts Changes in Insulin Sensitivity in Response to Long-Term Exercise. Physiol. Rep. 2017, 5, e13183.
- Di Paola, M.; Lorusso, M. Interaction of Free Fatty Acids with Mitochondria: Coupling, Uncoupling and Permeability Transition. Biochim. Biophys. Acta BBA Bioenerg. 2006, 1757, 1330–1337.
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755.
- Kma, L.; Baruah, T.J. The Interplay of ROS and the PI3K/Akt Pathway in Autophagy Regulation. Biotech. Appl. Biochem. 2022, 69, 248–264.
- McCarthy, J.J.; Murach, K.A. Anabolic and Catabolic Signaling Pathways That Regulate Skeletal Muscle Mass. In Nutrition and Enhanced Sports Performance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–290. ISBN 978-0-12-813922-6.
- Jang, Y.C.; Rodriguez, K.; Lustgarten, M.S.; Muller, F.L.; Bhattacharya, A.; Pierce, A.; Choi, J.J.; Lee, N.H.; Chaudhuri, A.; Richardson, A.G.; et al. Superoxide-Mediated Oxidative Stress Accelerates Skeletal Muscle Atrophy by Synchronous Activation of Proteolytic Systems. GeroScience 2020, 42, 1579–1591.
- Nader, G.A. Molecular Determinants of Skeletal Muscle Mass: Getting the “AKT” Together. Int. J. Biochem. Cell Biol. 2005, 37, 1985–1996.
- Kitaoka, Y. The Role of Nrf2 in Skeletal Muscle on Exercise Capacity. Antioxidants 2021, 10, 1712.
- Huang, C.-J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med. Open 2015, 1, 32.
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526.
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421.
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Bandinelli, S.; Ferrucci, L. Association of Adherence to the Mediterranean-Style Diet with Lower Frailty Index in Older Adults. Nutrients 2021, 13, 1129.
- Amiot, M.-J.; Latgé, C.; Plumey, L.; Raynal, S. Intake Estimation of Phytochemicals in a French Well-Balanced Diet. Nutrients 2021, 13, 3628.
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024.
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013, 2013, bat070.
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Phenol-Explorer 2.0: A Major Update of the Phenol-Explorer Database Integrating Data on Polyphenol Metabolism and Pharmacokinetics in Humans and Experimental Animals. Database 2012, 2012, bas031.
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445.
- Bernatoniene, J.; Kopustinskiene, D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965.
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-Dependent Signaling Pathways of Toll-like Receptor by (−)-Epigallocatechin-3-Gallate, a Polyphenol Component of Green Tea. Biochem. Pharmacol. 2006, 72, 850–859.
- Casanova, E.; Salvadó, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532.
- Li, Y.; Zhao, S.; Zhang, W.; Zhao, P.; He, B.; Wu, N.; Han, P. Epigallocatechin-3-O-Gallate (EGCG) Attenuates FFAs-Induced Peripheral Insulin Resistance through AMPK Pathway and Insulin Signaling Pathway in Vivo. Diabetes Res. Clin. Pract. 2011, 93, 205–214.
- Pallauf, K.; Rimbach, G. Autophagy, Polyphenols and Healthy Ageing. Ageing Res. Rev. 2013, 12, 237–252.
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, Y.; Zheng, P.; Yu, J.; He, J. Grape Seed Proanthocyanidin Extract Promotes Skeletal Muscle Fiber Type Transformation via AMPK Signaling Pathway. J. Nutr. Biochem. 2020, 84, 108462.
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796.
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. JIR 2020, 13, 421–431.
- Pandey, K.B.; Rizvi, S.I. Role of Red Grape Polyphenols as Antidiabetic Agents. Integr. Med. Res. 2014, 3, 119–125.
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073.
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811.
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods 2020, 9, 687.
- Jia, Y.; Wu, C.; Kim, Y.-S.; Yang, S.O.; Kim, Y.; Kim, J.-S.; Jeong, M.-Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A Dietary Anthocyanin Cyanidin-3-O-Glucoside Binds to PPARs to Regulate Glucose Metabolism and Insulin Sensitivity in Mice. Commun. Biol. 2020, 3, 514.
- Geng, X.; Ji, J.; Liu, Y.; Li, X.; Chen, Y.; Su, L.; Zhao, L. Cyanidin-3-O-Glucoside Supplementation Ameliorates Metabolic Insulin Resistance via Restoration of Nitric Oxide-Mediated Endothelial Insulin Transport. Mol. Nutr. Food Res. 2022, 66, 2100742.
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264.
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant Apigenin Relieves Age-Related Muscle Atrophy by Inhibiting Oxidative Stress and Hyperactive Mitophagy and Apoptosis in Skeletal Muscle of Mice. J. Gerontol. Ser. A 2020, 75, 2081–2088.
- Park, M.-H.; Ju, J.-W.; Park, M.; Han, J. Daidzein Inhibits Carbohydrate Digestive Enzymes in Vitro and Alleviates Postprandial Hyperglycemia in Diabetic Mice. Eur. J. Pharmacol. 2013, 712, 48–52.
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, Its Effects on Impaired Glucose and Lipid Metabolism and Vascular Inflammation Associated with Type 2 Diabetes: Prophylactic Role of Daidzein in Type 2 Diabetes. BioFactors 2018, 44, 407–417.
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein Stimulates Insulin Sensitivity through Gut Microbiota Reshaping and Skeletal Muscle AMPK Activation in Obese Subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948.
- Rehman, K.; Khan, I.I.; Akash, M.S.H.; Jabeen, K.; Haider, K. Naringenin Downregulates Inflammation-mediated Nitric Oxide Overproduction and Potentiates Endogenous Antioxidant Status during Hyperglycemia. J. Food Biochem. 2020, 44, e13422.
- Dhanya, R.; Jayamurthy, P. In Vitro Evaluation of Antidiabetic Potential of Hesperidin and Its Aglycone Hesperetin under Oxidative Stress in Skeletal Muscle Cell Line. Cell Biochem. Funct. 2020, 38, 419–427.
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122.
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.-J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.-J.; Lee, D.-H. Resveratrol Improves Insulin Signaling in a Tissue-Specific Manner under Insulin-Resistant Conditions Only: In Vitro and in Vivo Experiments in Rodents. Metabolism 2012, 61, 424–433.
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol Prevents Sarcopenic Obesity by Reversing Mitochondrial Dysfunction and Oxidative Stress via the PKA/LKB1/AMPK Pathway. Aging 2019, 11, 2217–2240.
- Bai, C.-H.; Alizargar, J.; Peng, C.-Y.; Wu, J.-P. Combination of Exercise Training and Resveratrol Attenuates Obese Sarcopenia in Skeletal Muscle Atrophy. Chin. J. Physiol. 2020, 63, 101.
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370.
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of Hyperglycemia and Dyslipidemia in Experimental Type 2 Diabetes by Gallic Acid and P-Coumaric Acid: The Role of Adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097.
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516.
- Jung, Y.; Park, J.; Kim, H.; Sim, J.; Youn, D.; Kang, J.; Lim, S.; Jeong, M.; Yang, W.M.; Lee, S.; et al. Vanillic Acid Attenuates Obesity via Activation of the AMPK Pathway and Thermogenic Factors in Vivo and in Vitro. FASEB J. 2018, 32, 1388–1402.
- Rashedinia, M.; Alimohammadi, M.; Shalfroushan, N.; Khoshnoud, M.J.; Mansourian, M.; Azarpira, N.; Sabahi, Z. Neuroprotective Effect of Syringic Acid by Modulation of Oxidative Stress and Mitochondrial Mass in Diabetic Rats. BioMed Res. Int. 2020, 2020, 8297984.
This entry is offline, you can click here to edit this entry!
