Non-invasive methods of detecting heat stress magnitude for livestock is gaining momentum in the context of global climate change. The concept of a non-invasive approach to assess heat stress primarily looks into behavioral and physiological responses which can be monitored without any human interference or additional stress on the animal. Bioclimatic thermal indices can be considered as the least invasive approach to assess and/or predict the level of heat stress in livestock. Assessing these responses can prove beneficial to quantifying heat stress and thereby enforcing suitable amelioration and mitigation strategies. There are a number of approaches to quantify heat stress, which in the current scenario with increasing animal welfare concern, can be considered as invasive and non-invasive approaches.
- heat stress
- animal welfare
- non-invasive
- IRT
- sensors
1. Methods to Quantify Heat Stress Response
1.1. Invasive Approaches to Quantify Heat Stress
1.2. Non-Invasive Approaches
2. Sensor-Based Applications in Assessing Heat Stress Response in Grazing Animals
2.1. Rumen/Reticular Boluses
2.2. Subcutaneous Implantable Devices
2.3. Rectal and Vaginal Probes
2.4. GPS Technology
2.5. Accelerometer
2.6. Bioacoustics
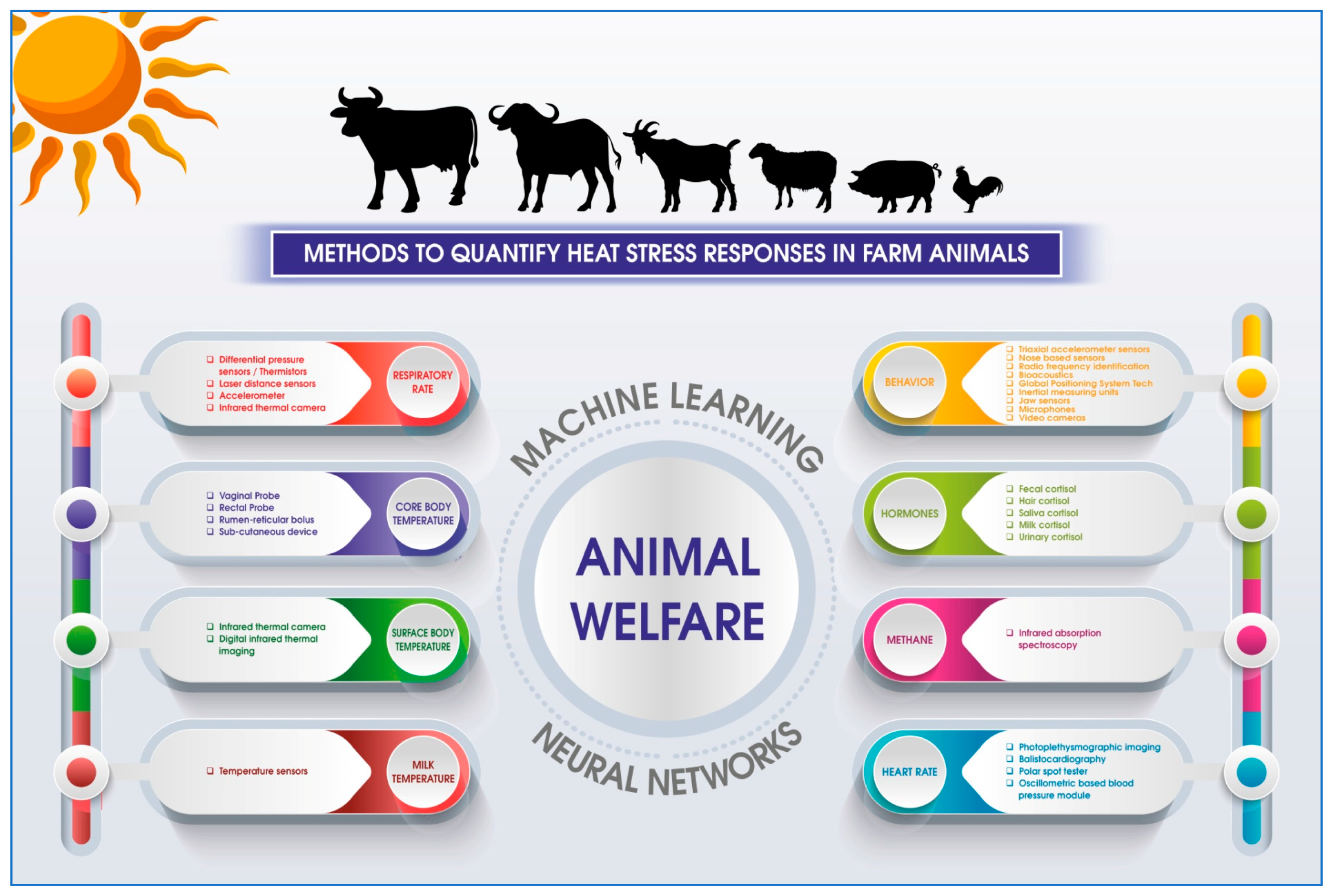
3. Applications of Machine Learning in Heat Stress Assessment in Farm Animals
This entry is adapted from the peer-reviewed paper 10.3390/atmos13101642
References
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71.
- Attia, N. Physiological, Hematological and Biochemical Alterations in Heat Stressed Goats. Benha Vet. Med. J. 2016, 31, 56–62.
- Morar, D.; Ciulan, V.; Simiz, F.; Moț, T.; Hutu, I.; Văduva, C. Effect of Heat Stress on Haematological Parameters in Dairy Cows. In Proceedings of the Lucrari Stiintifice: Medicina Veterinara Timisoara (Scientifical Papers: Veterinary Medicine Timisoara), Timisoara, Romania, 29 May 2018; Imprimeria Mirton Timisoara: Timisoara, Romania, 2018; Volume LI, pp. 65–70.
- Omran, F.I.; Ashour, G.; Youssef, M.M.; Shafie, M.M. Responses of Hematology, Blood Metabolites, Mineral Ions and Hormonal Profile to Heat Stress for Egyptian Buffalo-Calves. Egypt. J. Agric. Res. 2011, 89, 1129–1140.
- Chaudhary, S.S.; Singh, V.K.; Upadhyay, R.C.; Puri, G.; Odedara, A.B.; Patel, P.A. Evaluation of Physiological and Biochemical Responses in Different Seasons in Surti Buffaloes. Vet. World 2015, 8, 727–731.
- Aleena, J.; Sejian, V.; Krishnan, G.; Bagath, M.; Pragna, P.; Bhatta, R. Heat Stress Impact on Blood Biochemical Response and Plasma Aldosterone Level in Three Different Indigenous Goat Breeds. J. Anim. Behav. Biometeorol. 2020, 8, 266–275.
- Feizi, A.; Dadian, F.; Asadzadehmajdi, S. The Effect of Heat Stress on Some Blood Parameters, Biochemical Values and Humoral Immunity in Broiler Chickens. Vet. Clin. Pathol. Q. Sci. J. 2012, 6, 1621–1627.
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animal 2018, 12, s431–s444.
- Maurya, V.P.; Sejian, V.; Kumar, D.; Naqvi, S.M.K. Impact of Heat Stress, Nutritional Stress and Their Combinations on the Adaptive Capability of Malpura Sheep under Hot Semi-Arid Tropical Environment. J. Anim. Behav. Biometeorol. 2019, 7, 31–38.
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183.
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer Season Induced Rhythmic Alterations in Metabolic Activities to Adapt to Heat Stress in Three Indigenous (Osmanabadi, Malabari and Salem Black) Goat Breeds. Biol. Rhythm Res. 2018, 49, 551–565.
- Sejian, V.; Bagath, M.; Krishnan, G.; Rashamol, V.P.; Pragna, P.; Devaraj, C.; Bhatta, R. Genes for Resilience to Heat Stress in Small Ruminants: A Review. Small Rumin. Res. 2019, 173, 42–53.
- Manjari, R.; Yadav, M.; Ramesh, K.; Uniyal, S.; Rastogi, S.K.; Sejian, V.; Hyder, I. HSP70 as a Marker of Heat and Humidity Stress in Tarai Buffalo. Trop. Anim. Health Prod. 2015, 47, 111–116.
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression Patterns of Candidate Genes Reflecting the Growth Performance of Goats Subjected to Heat Stress. Mol. Biol. Rep. 2018, 45, 2847–2856.
- Archana, P.R.; Sejian, V.; Ruban, W.; Bagath, M.; Krishnan, G.; Aleena, J.; Manjunathareddy, G.B.; Beena, V.; Bhatta, R. Comparative Assessment of Heat Stress Induced Changes in Carcass Traits, Plasma Leptin Profile and Skeletal Muscle Myostatin and HSP70 Gene Expression Patterns between Indigenous Osmanabadi and Salem Black Goat Breeds. Meat Sci. 2018, 141, 66–80.
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Effect of Heat Stress on the Quantitative Expression Patterns of Different Cytokine Genes in Malabari Goats. Int. J. Biometeorol. 2019, 63, 1005–1013.
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46.
- Garner, J.B.; Chamberlain, A.J.; Vander Jagt, C.; Nguyen, T.T.T.; Mason, B.A.; Marett, L.C.; Leury, B.J.; Wales, W.J.; Hayes, B.J. Gene Expression of the Heat Stress Response in Bovine Peripheral White Blood Cells and Milk Somatic Cells in Vivo. Sci. Rep. 2020, 10, 19181.
- Halli, K.; Vanvanhossou, S.F.; Bohlouli, M.; König, S.; Yin, T. Identification of Candidate Genes on the Basis of SNP by Time-Lagged Heat Stress Interactions for Milk Production Traits in German Holstein Cattle. PLoS ONE 2021, 16, e0258216.
- Wijffels, G.; Sullivan, M.; Gaughan, J. Methods to Quantify Heat Stress in Ruminants: Current Status and Future Prospects. Methods 2021, 186, 3–13.
- Rhoad, A.O. The Iberia Heat Tolerance Test for Cattle. Trop. Agric. 1944, 21, 162–164.
- Rashamol, V.P.; Sejian, V.; Pragna, P.; Lees, A.M.; Bagath, M.; Krishnan, G.; Gaughan, J.B. Prediction Models, Assessment Methodologies and Biotechnological Tools to Quantify Heat Stress Response in Ruminant Livestock. Int. J. Biometeorol. 2019, 63, 1265–1281.
- Herbut, P.; Hoffmann, G.; Angrecka, S.; Godyń, D.; Vieira, F.M.C.; Adamczyk, K.; Kupczyński, R. The Effects of Heat Stress on the Behaviour of Dairy Cows—A Review. Ann. Anim. Sci. 2021, 21, 385–402.
- Aleena, J.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Resilience of Three Indigenous Goat Breeds to Heat Stress Based on Phenotypic Traits and PBMC HSP70 Expression. Int. J. Biometeorol. 2018, 62, 1995–2005.
- Allen, J.D.; Hall, L.W.; Collier, R.J.; Smith, J.F. Effect of Core Body Temperature, Time of Day, and Climate Conditions on Behavioral Patterns of Lactating Dairy Cows Experiencing Mild to Moderate Heat Stress. J. Dairy Sci. 2015, 98, 118–127.
- Islam, M.A.; Lomax, S.; Doughty, A.; Islam, M.; Jay, O.; Thomson, P.; Clark, C. Automated Monitoring of Cattle Heat Stress and Its Mitigation. Anim. Sci. 2021, 2, 737213.
- Herbut, P.; Angrecka, S. The Effect of Heat Stress on Time Spent Lying by Cows in a Housing System. Ann. Anim. Sci. 2018, 18, 825–833.
- Habibu, B.; Yaqub, L.S.; Dzenda, T.; Kawu, M.U. Sensitivity, Impact and Consequences of Changes in Respiratory Rate During Thermoregulation in Livestock—A Review. Ann. Anim. Sci. 2019, 19, 291–304.
- Pastell, M.; Kaihilahti, J.; Aisla, A.-M.; Hautala, M.; Poikalainen, V.; Ahokas, J. A System for Contact-Free Measurement of Respiration Rate of Dairy Cows. J. Prec. Livest. Farm 2007, 7, 105–109.
- Milan, H.F.M.; Maia, A.S.C.; Gebremedhin, K.G. Technical Note: Device for Measuring Respiration Rate of Cattle under Field Conditions1. J. Anim. Sci. 2016, 94, 5434–5438.
- Rees, A.; Fischer-Tenhagen, C.; Heuwieser, W. Effect of Heat Stress on Concentrations of Faecal Cortisol Metabolites in Dairy Cows. Reprod. Domest. Anim. 2016, 51, 392–399.
- Dulude-de Broin, F.; Côté, S.D.; Whiteside, D.P.; Mastromonaco, G.F. Faecal Metabolites and Hair Cortisol as Biological Markers of HPA-Axis Activity in the Rocky Mountain Goat. Gen. Comp. Endocrinol. 2019, 280, 147–157.
- Lewis Baida, B.E.; Swinbourne, A.M.; Barwick, J.; Leu, S.T.; van Wettere, W.H.E.J. Technologies for the Automated Collection of Heat Stress Data in Sheep. Anim. Biotelemetry 2021, 9, 4.
- Burdick, N.C.; Carroll, J.A.; Dailey, J.W.; Randel, R.D.; Falkenberg, S.M.; Schmidt, T.B. Development of a Self-Contained, Indwelling Vaginal Temperature Probe for Use in Cattle Research. J. Therm. Biol. 2012, 37, 339–343.
- Lees, A.M.; Lees, J.C.; Lisle, A.T.; Sullivan, M.L.; Gaughan, J.B. Effect of Heat Stress on Rumen Temperature of Three Breeds of Cattle. Int. J. Biometeorol. 2018, 62, 207–215.
- Torrao, N.A.; Hetem, R.S.; Meyer, L.C.R.; Fick, L.G. Assessment of the Use of Temperature-Sensitive Microchips to Determine Core Body Temperature in Goats. Vet. Rec. 2011, 168, 328.
- Taylor, D.B.; Schneider, D.A.; Brown, W.Y.; Price, I.R.; Trotter, M.G.; Lamb, D.W.; Hinch, G.N.; Taylor, D.B.; Schneider, D.A.; Brown, W.Y.; et al. GPS Observation of Shelter Utilisation by Merino Ewes. Anim. Prod. Sci. 2011, 51, 724–737.
- Koltes, J.E.; Koltes, D.A.; Mote, B.E.; Tucker, J.; Hubbell, D.S., III. Automated Collection of Heat Stress Data in Livestock: New Technologies and Opportunities. Transl. Anim. Sci. 2018, 2, 319–323.
- AlZahal, O.; AlZahal, H.; Steele, M.A.; Van Schaik, M.; Kyriazakis, I.; Duffield, T.F.; McBride, B.W. The Use of a Radiotelemetric Ruminal Bolus to Detect Body Temperature Changes in Lactating Dairy Cattle. J. Dairy Sci. 2011, 94, 3568–3574.
- Beatty, D.T.; Barnes, A.; Fleming, P.A.; Taylor, E.; Maloney, S.K. The Effect of Fleece on Core and Rumen Temperature in Sheep. J. Therm. Biol. 2008, 33, 437–443.
- Taylor, N.A.S.; Tipton, M.J.; Kenny, G.P. Considerations for the Measurement of Core, Skin and Mean Body Temperatures. J. Therm. Biol. 2014, 46, 72–101.
- Vickers, L.A.; Burfeind, O.; von Keyserlingk, M.A.G.; Veira, D.M.; Weary, D.M.; Heuwieser, W. Technical Note: Comparison of Rectal and Vaginal Temperatures in Lactating Dairy Cows. J. Dairy Sci. 2010, 93, 5246–5251.
- Pent, G.J.; Fike, J.H.; Kim, I. Ewe Lamb Vaginal Temperatures in Hardwood Silvopastures. Agrofor. Syst. 2021, 95, 21–32.
- Bailey, D.W.; Trotter, M.G.; Knight, C.W.; Thomas, M.G. Use of GPS Tracking Collars and Accelerometers for Rangeland Livestock Production Research1. Transl. Anim. Sci. 2018, 2, 81–88.
- Yang, C.-C.; Hsu, Y.-L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010, 10, 7772–7788.
- Umstätter, C.; Waterhouse, A.; Holland, J.P. An Automated Sensor-Based Method of Simple Behavioural Classification of Sheep in Extensive Systems. Comput. Electron. Agric. 2008, 64, 19–26.
- Corkery, G.; Ward, S.; Kenny, C.; Hemmingway, P. Incorporating Smart Sensing Technologies into the Poultry Industry. J. Worlds Poult. Res. 2013, 3, 106–128.
- IBM. What Is Machine Learning? Available online: https://www.ibm.com/in-en/cloud/learn/machine-learning (accessed on 16 September 2022).
- Domingos, P. A Few Useful Things to Know about Machine Learning. Commun. ACM 2012, 55, 78–87.
- García, R.; Aguilar, J.; Toro, M.; Pinto, A.; Rodríguez, P. A Systematic Literature Review on the Use of Machine Learning in Precision Livestock Farming. Comput. Electron. Agric. 2020, 179, 105826.
- Banhazi, T.M.; Lehr, H.; Black, J.L.; Crabtree, H.; Schofield, P.; Tscharke, M.; Berckmans, D. Precision Livestock Farming: An International Review of Scientific and Commercial Aspects. Int. J. Agric. Biol. Eng. 2012, 5, 1–9.
- Kotsiantis, S.B. Supervised Machine Learning: A Review of Classification Techniques. In Proceedings of the 2007 Conference on Emerging Artificial Intelligence Applications in Computer Engineering: Real Word AI Systems with Applications in eHealth, HCI, Information Retrieval and Pervasive Technologies, 10 June 2007; IOS Press: Amsterdam, The Netherlands; pp. 3–24.
- Ghafouri-Kesbi, F.; Rahimi-Mianji, G.; Honarvar, M.; Nejati-Javaremi, A.; Ghafouri-Kesbi, F.; Rahimi-Mianji, G.; Honarvar, M.; Nejati-Javaremi, A. Predictive Ability of Random Forests, Boosting, Support Vector Machines and Genomic Best Linear Unbiased Prediction in Different Scenarios of Genomic Evaluation. Anim. Prod. Sci. 2016, 57, 229–236.
- White, B.J.; Amrine, D.E.; Larson, R.L. Big data analytics and precision animal agriculture symposium: Data to Decisions. J. Anim. Sci. 2018, 96, 1531–1539.
- Libbrecht, M.W.; Noble, W.S. Machine Learning Applications in Genetics and Genomics. Nat. Rev. Genet. 2015, 16, 321–332.
- Neethirajan, S. Transforming the Adaptation Physiology of Farm Animals through Sensors. Animals 2020, 10, 1512.
- Williams, M.L.; Mac Parthaláin, N.; Brewer, P.; James, W.P.J.; Rose, M.T. A Novel Behavioral Model of the Pasture-Based Dairy Cow from GPS Data Using Data Mining and Machine Learning Techniques. J. Dairy Sci. 2016, 99, 2063–2075.
- Benaissa, S.; Tuyttens, F.A.M.; Plets, D.; de Pessemier, T.; Trogh, J.; Tanghe, E.; Martens, L.; Vandaele, L.; Van Nuffel, A.; Joseph, W.; et al. On the Use of On-Cow Accelerometers for the Classification of Behaviours in Dairy Barns. Res. Vet. Sci. 2019, 125, 425–433.
- Guo, Y.; He, D.; Chai, L. A Machine Vision-Based Method for Monitoring Scene-Interactive Behaviors of Dairy Calf. Animals 2020, 10, 190.
- Gorczyca, M.T. Machine Learning Applications for Monitoring Heat Stress in Livestock. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2019.
- Gorczyca, M.T.; Gebremedhin, K.G. Ranking of Environmental Heat Stressors for Dairy Cows Using Machine Learning Algorithms. Comput. Electron. Agric. 2020, 168, 105124.
- Kim, S.; Hidaka, Y. Breathing Pattern Analysis in Cattle Using Infrared Thermography and Computer Vision. Animals 2021, 11, 207.
- De Sousa, R.V.; da Rodrigues, A.V.S.; de Abreu, M.G.; Tabile, R.A.; Martello, L.S. Predictive Model Based on Artificial Neural Network for Assessing Beef Cattle Thermal Stress Using Weather and Physiological Variables. Comput. Electron. Agric. 2018, 144, 37–43.
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest. Sci. 2010, 130, 57–69.
- Benni, S.; Pastell, M.; Bonora, F.; Tassinari, P.; Torreggiani, D. A Generalised Additive Model to Characterise Dairy Cows’ Responses to Heat Stress. Animal 2020, 14, 418–424.
- Bovo, M.; Agrusti, M.; Benni, S.; Torreggiani, D.; Tassinari, P. Random Forest Modelling of Milk Yield of Dairy Cows under Heat Stress Conditions. Animals 2021, 11, 1305.
