Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Stereotactic ablative radiation therapy (SAbR) is a safe and effective local therapy for renal cell cancer (RCC) with emerging and evolving indications. SAbR has been demonstrated to be safe and effective in providing local control to both primary and metastatic RCC by using ablative radiation doses. SAbR can be integrated with other local and systemic therapies to provide optimal management of RCC patients.
- SBRT
- SAbR
- radiation
- oligometastasis
- oligoprogression
1. SAbR for Primary RCC
The standard curative treatment for primary RCC is surgery, however patient characteristics such as inoperability or tumor size may favor other local treatments or observation. Other local therapies for primary RCC such as radiofrequency and cryoablations are invasive procedures with limitations based on size and location of the tumor, unlike non-invasive SAbR. Among the earliest outcomes to show promising results of SAbR for primary RCC are several retrospective studies published in the mid-2000s [20,23,24]. The first prospective dose escalation trials of SAbR showed that doses >27 Gy in 3 fractions did not have any failures and reported an overall LC of 93.7%, with failures only happening in earlier cohorts that received <10 Gy per each treatment [25] and escalation to 48 Gy in 4 fractions was achieved without dose-limiting toxicities [26]. Interestingly, lesions treated with SAbR decreased in size, however the tumor enhancement did not change on contrast imaging, suggesting that while the tumor cells were killed, the vasculature in the lesion was not affected. Another phase 2 trial of 37 patients with localized RCC treated with SAbR reported a LC of 100% at a median follow up of 24 months [27]. These authors also reported 3% grade 3 toxicity with no grade 4 to 5 toxicities.
2. Locally Advanced RCC
Standard of care treatment for patients with locally advanced RCC has traditionally been radical or partial nephrectomy. Postoperative treatment options have included observation and systemic therapy. More recently, investigators are exploring an increasingly nuanced approach given a variety of patient factors and outcomes.
SAbR may have application in the management of the RCC IVC tumor thrombus. An early case report of two patients treated with preoperative SAbR showed a median survival of 20 months at the time of publication, and no acute or late treatment-related toxicity [32]. A 15 patient retrospective, multi-institutional study reported a 58% response rate of RCC IVC tumor thrombus after SAbR, with symptomatic palliation in all patients and only grade 1 to 2 toxicity [34]. There are numerous potential indications for SAbR of IVC tumor thrombus, such as: palliation of Budd-Chiari syndrome; unresectable or recurrent disease after surgery; disease refractory to surgery and systemic therapy; cytoreduction with systemic therapy to increase resectability by alleviation of Budd-Chiari/hepatic venous congestion, which significantly improves surgical mortality; preoperative MRI evidence of IVC wall invasion; and patient eligibility for radical nephrectomy, but not tumor thrombectomy. Given the higher incidence of RCC in elderly and comorbid patients, it is conceivable that there will be many patients with RCC IVC tumor thrombus who would be candidates for the much less risky radical nephrectomy, but not a high-risk vascular surgery of tumor thrombectomy, particularly when cardio-pulmonary bypass may be required. In this setting, a reasonable option may be to treat the IVC tumor thrombus with SAbR combined with a simple radical nephrectomy. Another advantage of this strategy is that it can be performed in the local and community hospitals, whereas IVC tumor thrombectomies are usually limited to high-volume tertiary academic centers.
Preoperative SAbR to the RCC IVC tumor thrombus is currently being investigated to reduce the high risk of recurrence. A safety lead-in phase II clinical trial of neoadjuvant SAbR for RCC IVC tumor thrombus (NCT02473536) is ongoing (Figure 1). The safety lead-in phase of the trial demonstrated that this treatment approach is feasible and safe, however, the oncologic outcome data is not yet complete [35]. This paradigm continues to evolve, and prospective evidence is currently lacking.
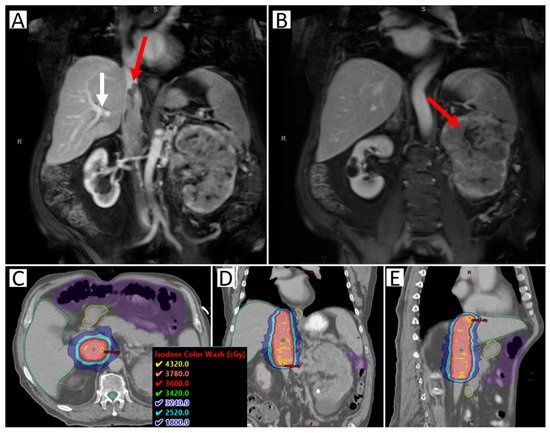
Figure 1. Images of a sample case of a patient with RCC IVC tumor thrombus level III, making resection not possible. The patient was treated with SAbR 36 Gy/3 fractions. (A) Coronal abdominal MR with contrast; red arrow highlighting the superior extent of the tumor thrombus; white arrow highlighting tumor thrombus extension beyond the branching of the hepatic artery (blockage the leads to Budd-Chiari Syndrome, making surgery more complicated). (B) Coronal abdominal MR with contrast; right arrow highlighting the large left primary kidney tumor. (C–E) Axial, coronal, and sagittal CT with radiation dose distribution shown. Nearby organ at risk highlighted include liver (green), duodenum (yellow), bowel space (purple), and spinal cord (green).
Consolidative or debulking SAbR may have applications in additional clinical scenarios. For instance, patients with locally advanced RCC without tumor thrombus may also be unresectable due to the extent of disease, medical inoperability, surgical risks, or simply due to a lack of evidence of clinical benefit as demonstrated by multiple clinical trials [36]. Singh et al. published feasibility data from a Phase 1 study that treated large kidney tumors with neoadjuvant SAbR [37]. Furthermore, patients initially diagnosed with metastatic disease may achieve a near complete response with systemic therapy, with only the primary tumor remaining. This could provide another scenario where SAbR can be utilized. There are ongoing multi-center phase 2 clinical trials (CYTOSHRINK NCT04090710 and SAMURAI NCT05327686) evaluating this strategy, leveraging potential synergy of SAbR with immunotherapy.
3. Oligometastatic RCC
Metastatic RCC represents an array of disease aggressiveness. Patients with International Metastatic Database Consortium (IMDC) low-risk disease may have a smoldering progression over many years, while patients with high-risk disease may die from their cancer in less than one year [38,39]. Additionally, metastatic RCC includes the range of patients with few involved sites to those with widely disseminated disease. Oligometastatic RCC can be divided into subcategories based on the risk of distant micrometastasis. These subcategories can be helpful to assess the probability of future progression at distant sites and the speed of progression.
Active surveillance is one treatment approach for appropriately selected indolent patients with oligometastatic RCC. A prospective trial of patients with oligometastatic RCC with proven indolent growth of metastases after primary nephrectomy showed that this subset of patients could safely undergo active surveillance for a median of 14.9 months before starting systemic therapy [39].
Metastasectomy is also a treatment option for patients with oligometastatic RCC, however data on surgical LC and safety are lacking [41]. A Japanese retrospective study of 1463 patients in which 20.8% underwent metastasectomy reported prognostic factors for metastatic RCC, including performance status, Hgb, LHD, serum calcium, C-reactive protein, and time from initial visit to metastasis being less than one year. Median survival for patients with no risk factors and one to two risk factors was 55.3 months and 29.6 months, respectively (one-year OS 92.8% and 76.6%, respectively) [45]. More recently, Tosco et al. investigated the survival impact of prognostic factors in patients with metastatic RCC who underwent metastasectomy [46]. Their results indicated that advanced primary tumor stage, high tumor grade, non-pulmonary metastases, disease-free interval of less than 12 months, and multi-organ metastases were independent factors for survival. Patients with 0 to 1, 2, 3, or greater than 4 factors had two-year cancer-specific survival rates of 95.8%, 89.9%, 65.6%, and 24.7%, respectively [46]. These tools may help clinical decision making for appropriate patient selection for local therapy.
SAbR is a promising local treatment option that has not only shown favorable LC rates of greater than 90%, but can also provide an option for local therapy at an otherwise inoperable location. A phase II prospective trial from Sweden used SAbR in primary and metastatic RCC and showed an OS of 32 months with a LC rate of 79% at a median follow-up of 52 months [20]. Additionally, the University of Chicago published a prospective study with 81% of initial metastatic progression in patients with oligometastatic RCC limited to less than five sites after treatment with SAbR. This study also confirmed that approximately half of patients had either no or limited metastatic progression after a median follow up of 20.9 months [47]. Aggressive upfront sequential SAbR is supported by these experiences as an effective local therapy that can potentially control disease progression in patients with limited metastases. Retrospective analyses have supported the use of SAbR for oligometastatic disease to defer the start of systemic therapy and possibly extend survival [42]. This has recently become the subject of prospective studies, including one that supported the efficacy and safety of this approach with SAbR [48]. Moreover, this strategy can provide durable disease control in the setting of additional oligometastatic lesions with sequential, subsequent focal SAbR. This approach was described in a retrospective study where 30% of patients received two or more courses of SAbR to additional sites of metastatic disease [42]. The first phase II trial demonstrating the efficacy of sequential SAbR in the control of systemic therapy naïve oligometastatic RCC reported a one-year progression-free interval of 82.6%, and a one-year freedom from systemic therapy of 91.3% with no measured decline in patient-reported quality of life [44,49]. Tang et al. published a prospective feasibility phase II study where subsequent sites of progression were allowed to be treated with SAbR and found a median PFS of 22.7 months with acceptable toxicity [43]. While the study met its feasibility endpoint, it did not meet its estimated efficacy endpoint of 71% one-year PFS and reported a one-year PFS of 64%.
While the safety of SAbR has been excellent in published series, caution must be exercised in certain scenarios. For instance, due to the vascular nature of RCC, ultra-central lung metastasis have rarely been shown to be associated with serious life-threatening late effects such as hemoptysis or hemothorax years after treatment. It is often difficult in these situations to assess the contribution of radiation, tumor recurrence, and systemic therapy as the etiology of the hemoptysis. A second potential cautionary scenario is the accentuation of future systemic therapy toxicities (i.e., colitis or pneumonitis), or a radiation-recall-type side effect.
4. Oligoprogressive RCC
Individuals with metastatic RCC can develop disease progression at only a few select sites of disease, deemed oligoprogressive (Figure 2). To date, there has been limited research on patterns of progression. For example, conventionally used criteria for response assessment in clinical trials, such as Response Evaluation Criteria in Solid Tumors (RECIST) criteria, do not distinguish patterns of progression. The current approach to progression in clinical practice is to switch systemic therapy, even if progression is limited to a few sites. This is also an approach for patients who are otherwise tolerating the ongoing systemic therapy well. There are likely different modes of progression that reflect a spectrum of disease responsiveness to therapy and cancer biology. One hypothesis to explain limited disease progression while on systemic therapy is mutational heterogeneity and clonally propagated branched evolution that fosters tumor adaptation and therapeutic failure through Darwinian selection [50,51,52]. This spectrum of disease progression may thus be optimally managed with a strategy different from the singular standard approach to change systemic therapy.
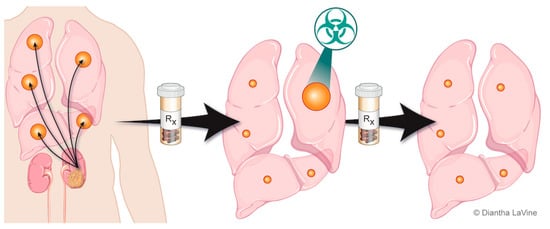
Figure 2. Depiction of oligoprogressive metastatic RCC. The metastatic disease is treated with systemic therapy and has a favorable response except at limited site(s). The limited progressive disease can be targeted with radiation therapy (SAbR), which provides durable control so the patient can remain on the same systemic therapy.
The introduction of focal therapies for controlling oligoprogressive sites (Figure 2) could be advantageous by increasing duration of the current therapy and preserving the limited available subsequent therapies. By extending duration of the current systemic therapy and altering the course of the disease through elimination of resistant metastasis, it is possible that this approach could also improve survival outcomes. In addition, reported subsequent lines of systemic therapy are typically associated with shorter PFS intervals and are often associated with increased toxicity [53]. Local therapy seems unlikely to undermine future systemic therapy, and such an approach may extend patient survival.
SAbR for oligoprogressive mRCC has been shown to be generally well-tolerated. Toxicity may also be exacerbated, however, by both ICIs and TKIs, and the safety of SAbR in conjunction with systemic therapy continues to be evaluated. SAbR with concurrent ICI/TKI was started with caution due to concerns for potential increased toxicity, but no enhanced toxicity was observed in initial studies [63,64,65]. This warrants the need for further prospective studies in this space. Mohamad et al. evaluated the safety of concurrent ICI and hypofractionated radiotherapy in 59 patients with mRCC, and concluded that adverse events of any grade did not significantly differ from historical rates of ICI therapy alone [66]. In a phase I trial, Tang et al. treated 55 patients with ipilimumab and either concurrent or sequential SAbR. They reported a 34% rate of grade 3 toxicity, comparable to treatment with ipilimumab alone [67]. Contrastingly, a meta-analysis of 13 prospective randomized trials with concurrent TKI and radiation therapy showed increased grade 3 or greater toxicity [68]. However, a different pooled analysis of 68 prospective trials of ICIs showed that those who received an ICI within 90 days following radiation therapy did not appear to be associated with an increased risk of serious adverse events [69].
5. CNS and Spine Metastasis
Brain metastases have been reported in up to 17% of patients with RCC [70]. With the recent approval of more effective systemic therapies, patients with mRCC live longer, and there is an expected increase in incidence of brain metastases for these patients [4,6,7,8]. Despite improvements in systemic therapies, the blood–brain barrier poses a persistent challenge to treat RCC brain metastases. This is why local therapy, such as surgery or radiation, remains a crucial treatment option for treating brain metastases [71]. Surgical resection has been a traditional treatment approach, however that may not always be possible due to patient or tumor factors. Classical radiation treatment for intracranial metastases has generally involved whole-brain radiation therapy (WBRT); however, this paradigm has shifted to prefer stereotactic radio surgery (SRS). SRS for RCC-specific brain metastases also allows greater dose-per fraction treatments to combat what has traditionally considered a radioresistant histology. SRS has less neurocognitive toxicity without a survival detriment compared to WBRT with SRS [72]. LC rates have been excellent, reaching as high as 98% to 100% in certain series [70,73,74,75,76]. SRS not only has significant clinical advantages, but there are logistical benefits as well. SRS is a minimally invasive outpatient procedure, it can be performed on patients unfit for surgery, and is feasible in certain intracranial locations deemed unresectable.
SAbR can increase the risk of vertebral compression fracture further, and it is therefore recommended to pursue prophylactic kyphoplasty [83]. Surgical resection for RCC metastasis also poses an intraoperative bleeding risk that can be addressed with arterial embolization prior to resection. Consequentially, a multi-disciplinary approach is ideal for the proper management of spinal metastasis from RCC.
6. Palliation
Aside from the previously outlined setting of CNS, there are various additional scenarios where SAbR may be indicated for the treatment of RCC with curative, consolidative, and adjuvant intent. Additionally, multiple indications for palliative irradiation for RCC also exist. The most common sites of metastatic disease in patients with RCC have been documented as: lung (45%), bone (30%), lymph node (22%), liver (20%), brain (9%), and adrenal (9%) [84]. Indications for palliative radiation include radiologic evidence of metastatic disease and a corresponding sign or symptom such as pain, spinal cord compression, superior vena cava syndrome, brain metastasis, fracture and prevention of fracture in the weight bearing bones, bleeding, and more. Hematuria is a frequent presenting symptom for metastatic RCC that can be palliated effectively with radiation therapy [85]. Due to the classic radio-resistance of RCC to conventional fractionation, hypofractionation schemes favoring a higher dose per fraction are preferred. The regimen of 20 Gy in 5 fractions is preferred over 30 Gy in 10 fractions. Whenever possible, applicable dose escalation should be considered with intensity-modulated radiation therapy or SAbR.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14194693
This entry is offline, you can click here to edit this entry!
