Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Ceramics have become indispensable materials for a wide range of industrial applications due to their excellent properties.
- ceramics
- polymer-derived ceramics technology
- structure and properties
1. Introduction
In the 1960s, Ainger and Chanttrell obtained non-oxide ceramics for the first time by pyrolyzing polymer precursors [1][2]; this method laid the foundation for the development of polymer-derived ceramics (PDCs). Later, in the early 1970s, Verbeek and colleagues successfully produced Si3N4/SiC ceramic fibers from precursors, such as polycarbosilanes, polysilanes, and polysiloxanes [3][4]. Then, in the mid-1970s, Fritz and Yajima [5][6] pyrolyzed polycarbosilane to obtain SiC ceramics; this technique greatly promoted the development of PDCs. Since then, PDCs have gradually been employed by scientists and engineers due to their advantages, such as excellent oxidation, creep, ablation, and crystallization resistances, as well as high-temperature stability [7][8].
High-entropy ceramics (HECs) are a class of novel materials with high configurational entropy (ΔSconfig > 1.5 R) [9]. The first single-phase HECs were synthesized by Rost and co-workers in 2015 [9], and several HECs with different crystal structures have been produced to date [10][11][12][13]. Research on HECs has revealed their extraordinary properties, including mechanical, electrical, and corrosion resistance [14][15][16], which have expanded their application prospects. The process of preparing PDCs from polymer precursors is referred to as PDCs technology. In recent years, several attempts have been made to prepare high-entropy ceramics using PDCs technology, with some success [17][18][19]. These advances show a new way of understanding and discovering high-entropy ceramics.
2. Polymer-Derived Ceramics Technology
At present, most of the precursors of commercial PDCs are silicon-based ceramics precursors and their modified products, and, as PDCs technology has attracted more attention, researchers have prepared precursors not only from polymers but also precursors containing a variety of organic and inorganic components that require chemical reactions to obtain ceramic products [20]. Although there are more and more types of precursors, the characteristics and preparation procedure via PDCs technology are the same.
2.1. Characteristics of Polymer-Derived Ceramics Technology
The characteristics of the preparation process of PDCs technology and the improvement of the material properties via this method can be summarized as follows:
- i.
-
The designability of the organic precursor structure can be used to tune the microstructure of ceramics; in other words, the structure, composition, and preparation process of the organic polymer precursor are adjusted to control the phase composition and structure of the final ceramic product [21].
- ii.
-
The polymer precursors have good moldability and can be used to achieve the preparation of ceramics with complex shapes, including one-dimensional ceramic fibers [6], two-dimensional coatings [22], as well as three-dimensional micro-electro-mechanical systems (MEMS) [23] and ceramic composites [24]. The preparation of fibers takes advantage of the fusible nature of precursors [6][25]; the synthesis of the coating exploits the fluidity of the precursor to achieve a two-dimensional uniform structure on the surface of the material [26][27][28]. Polymer-derived ceramics technology can be applied in semiconductor preparation techniques, such as lithography, and in the synthesis of ceramic micro–nano devices through the design of the functional groups of the polymer, which provides a good processing route for the manufacture of MEMS [23]. The solubility of the precursor can also be used to impregnate the fiber precast [29][30][31]; after impregnation, the polymer is crosslinked, cured, and pyrolyzed at high-temperature into the ceramic matrix to fill voids in the precast (this preparation process is called PIP); after repeating the PIP process, a dense fiber-reinforced ceramic matrix composite is obtained.
- iii.
-
The process temperature is relatively low. Traditional non-oxide ceramics, such as SiC and Si3N4, require a high sintering temperature, usually above 1600 °C, while PDCs can be sintered at temperatures as low as 900 °C [32].
- iv.
-
Sintering aids are not needed. Due to the slow atomic diffusion caused by the properties of covalent bonds, sintering additives are often required in the preparation of non-oxide ceramics [33]. These additives form a liquid phase at high temperatures and accelerate the diffusion of atoms, thereby promoting the sintering of non-oxide ceramics [34][35]. However, the sintering additive residues at grain boundaries will impair the oxidation resistance [36][37] and the high-temperature mechanical properties of non-oxide ceramics (such as the high-temperature creep resistance) [38]. In contrast, PDCs technology can achieve the sintering of ceramic materials without sintering additives [39][40], and the resulting materials exhibit good resistance to high-temperature oxidation [41], as well as high-temperature creep properties [42].
- v.
-
Excellent high-temperature performance. Since no sintering additives are required in the preparation of polymer-derived ceramics, a high-purity matrix is obtained after sintering; thus, the prepared material has good high-temperature properties, such as creep [42][43][44], oxidation [41][45], and corrosion [46][47] resistances.
2.2. Procedure of Polymer-Derived Ceramics Technology
The most commonly used commercial precursors are silicon-based polymers, which include polycarbosilane, polysiloxane, polysilazane, and polyborosilazane and their modified products [20]. Thus, taking organosilicon as example, the processes of polymer-derived ceramics technology are as follows (Figure 1):
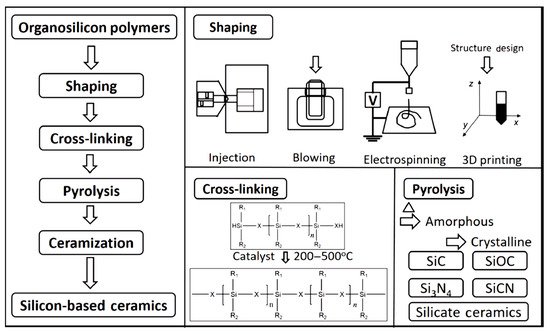
Figure 1. Preparation of ceramic products by PDCs technology [48].
- i.
-
Synthesis: various small organic molecules are used as raw materials to obtain precursors with specific molecular weights by organic synthesis methods [20]. The precursor can be varied by selecting suitable small molecules and optimizing the synthesis process. Ceramics with different microstructures can be obtained by using different precursors, as well as different curing and cracking systems [45][46][47][49][50].
- ii.
-
Shaping: polymers can be shaped directly with a variety of methods, such as injection molding, blow molding, extrusion molding, coating, electrospinning, 3D printing, etc., which further enable one-step molding of polymer-derived ceramics [48].
- iii.
-
Crosslinking/curing: the main purpose of crosslinking is to make the polymer backbone connected [20]. Crosslinking methods include light and thermal curing processes. Thermal-curing crosslinking generally relies on curing agents to polymerize polymer precursors into a mesh structure at a certain temperature, forming a non-molten polymer [51]. In light-curing crosslinking, a polymer is doped with a curing agent and polymerized under illumination at a specific wavelength to obtain a non-molten polymer [52].
- iv.
-
Pyrolysis/caramelization: these processes complete the transformation of the material from organic to inorganic, inducing qualitative changes in its internal structure and properties [20]. During the process, the organic groups of the precursor gradually vanish, and the polymer transforms into amorphous ceramics, with a typical pyrolyzing temperature of 900–1000 °C [48]. The phase composition, structure, and properties of amorphous ceramics obtained by pyrolysis are strongly dependent on the caramelization process.
- v.
-
Crystallization: typically, the polymer transforms into amorphous ceramics at a temperature between 900 and 1000 °C [38]. As the heat treatment temperature increases, the amorphous phase is gradually crystallized in the temperature range of 1200–1800 °C, and the crystalline ceramic material is finally obtained [20][38]. Several structural transformations are triggered by the amorphous → crystalline transition [20][29]: the amorphous disordered structure is rearranged with the relevant chemical bonds broken, and the structure gradually turns into crystalline as the temperature is increased; then, the rupture of chemical bonds and the atomic rearrangement cause the separation of the ceramic and carbon phases to form a multiphase ceramic system, which, in turn, promotes nucleation; the formed crystal nuclei gradually grow with increasing temperature and time. Take the C-enriched SiC produced by PDCs technology as an example; the amorphous → crystalline transition can be schematically drawn in Figure 2 [53]. Meanwhile, the amorphous → crystalline transition is usually accompanied by a decomposition reaction, along with the formation of a small amount of gaseous products.
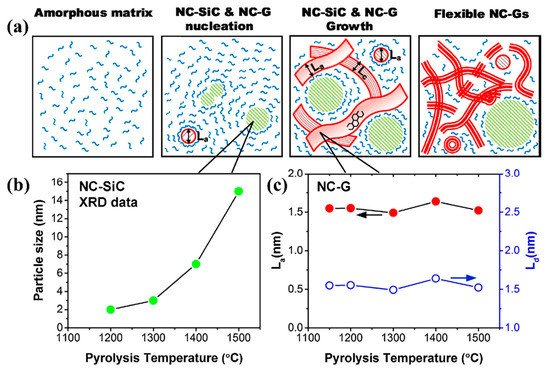 Figure 2. (a) Schematics of a detailed model describing the temperature-dependent evolution of nanodomains comprised of SiC, free carbon, and residual amorphous matrix in polymer-derived C-enriched SiC ceramics. (b) the average NC–SiC particle size varied with temperature, from 2 nm (1200 °C) to 15 nm (1500 °C). (c) the in-plane crystallite diameters (La) of the NC-G phase and the average defect distances of a single graphene sheet (Ld) grow trend varied with temperature, both remained nearly constant [54].
Figure 2. (a) Schematics of a detailed model describing the temperature-dependent evolution of nanodomains comprised of SiC, free carbon, and residual amorphous matrix in polymer-derived C-enriched SiC ceramics. (b) the average NC–SiC particle size varied with temperature, from 2 nm (1200 °C) to 15 nm (1500 °C). (c) the in-plane crystallite diameters (La) of the NC-G phase and the average defect distances of a single graphene sheet (Ld) grow trend varied with temperature, both remained nearly constant [54].
In addition, in order to introduce metallic elements into the backbone of the precursor to improve the thermal stability of PDCs, some researchers have also developed inorganic precursors [55]. For example, the most common inorganic precursor is metal chlorides, which are often used to synthesize polymetallosiloxanes with silicic acid and partially hydrolyzed tetraethoxysilanes [56]. After the synthesis of polymetallosiloxanes, taking the SiZrOC ceramics precursor as an example, the subsequent crosslinking process can retain the metal atoms in the backbone structure of the final product and then pyrolyze to obtain PDCs containing metal elements [57]. The formation of Si–O–Zr bonds occurs during crosslinking, as shown below [55]:
≡Si−H+≡Zr−OPr→≡Si−O−Zr≡+C3H8≡Si−H+≡Zr−OH→≡Si−O−Zr≡+H2
Ceramics prepared by PDCs technology undergo a unique phase evolution process with increasing temperature, and the performance of the final product is directly affected by the pyrolyzing process, precursor composition, and molecular structure (a comparison with the traditional manufacturing procedures is displayed in Table 1). Therefore, PDCs technology can provide control of the ceramic microstructure and properties, and it represents an innovative preparation process that will play an important role in industrial applications of ceramics in the future.
Table 1. Main processing parameters for manufacturing ceramics via conventional route and PDC route [58].
| Processing Parameters | Conventional Route | PDC Route |
|---|---|---|
| Ceramic Raw Material | Ceramic powders, such as alumina, zirconia, silicon carbide, or aluminum nitride | Precursor polymers, such as polysiloxanes or polysilazanes, with passive or active fillers |
| Mixing/Milling | Powders are mixed, generally in a ball mill, to liquid + dispersant for breaking up agglomerates; binders and plasticizers are added homogenized | Synthesis: solid or liquid are dissolved, with the aid of different equipment, in a solvent; fillers, crosslinkers, and others are added and homogenized |
| Shaping | Cutting or press into desired shapes | |
| Thermal Treatments | Debinding at middle temperatures and sintering at high temperatures are needed | Crosslinking at low temperatures (as low as room temperature) and pyrolysis at high temperatures are needed; eventually, crystallization at higher temperatures is accomplished; composite materials may be produced with partial pyrolysis of precursors |
| Ceramic Products | Dense parts with a residual porosity and controlled shrinkage, or, less often, macroporous parts; all kinds of oxide and non-oxide ceramics may be fabricated | Near net shape parts with the use of active/passive fillers, or controlled porosity with the aid of pore formers; mostly silicon-based ceramics are fabricated (SiC, SiOC, SiOCN…) |
This entry is adapted from the peer-reviewed paper 10.3390/cryst12091292
References
- Ainger, F.W.; Herbert, J.M. The Preparation of Phosphorus–Nitrogen Compounds as Non–Porous Solids; Academic Press: New York, NY, USA, 1960; pp. 168–182.
- Chantrell, P.G.; Popper, P. Inorganic Polymers and Ceramic; Academic Press: New York, NY, USA, 1960; pp. 87–103.
- Verbeek, W. Production of Shaped Articles of Homogeneous Mixtures of Silicon Carbide and Nitride. U.S. Patent 3853567, 8 November 1973.
- Verbeek, W.; Winter, G. Formkoerper aus siliciumcarbid und verfahren zu ihrer herstellung. Ger. Offen 1974, 7, 2236078.
- Fritz, G.; Raabe, B. Bildung siliciumorganischer verbindungen. v. die thermische zersetzung von Si(CH3)4 und Si(C2H5)4. Z. Anorg. Allg. Chem. 1956, 286, 149–167.
- Yajima, S.; Hayashi, J.; Imori, M. Continuous silicon carbide fiber of high tensile strength. Chem. Lett. 1975, 4, 931–934.
- Xia, A.; Yin, J.; Chen, X.; Liu, X.; Huang, Z. Polymer-Derived Si-Based Ceramics: Recent Developments and Perspectives. Crystals 2020, 10, 824.
- Liu, H.; Tian, C. Manufacturing Process of New Structural Ceramics-Polymer-Derived Method and its Application; China Machine Press: Beijing, China, 2010; pp. 11–12.
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Comm. 2015, 6, 8485.
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scripta Mater. 2018, 142, 116–120.
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskites type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327.
- Chen, J.; Liu, W.; Liu, J.; Zhang, X.; Yuan, M.; Zhao, Y.; Yan, J.; Hou, M.; Yan, J.; Kunz, M.; et al. Stability and Compressibility of cation-doped high-entropy oxide MgCoNiCuZnO5. J. Phys. Chem. C. 2019, 123, 17735–17744.
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109.
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys—A new era of exploitation. Mater. Sci. Forum 2007, 560, 1–9.
- Yeh, J.W. Recent progress in high-entropy alloys. Eur. J. Control. 2006, 33, 633–648.
- Ren, K.; Wang, Q.K.; Shao, G.; Zhao, X.F.; Wang, Y.G. Multicomponent high-entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scripta Mater. 2020, 178, 382–386.
- Ren, K.; Wang, Q.K.; Cao, Y.J.; Shao, G.; Wang, Y.G. Multicomponent rare-earth cerate and zirconocerate ceramics for thermal barrier coating materials. J. Eur. Ceram. Soc. 2021, 41, 1720–1725.
- Liu, J.; Ren, K.; Ma, C.Y.; Du, H.L.; Wang, Y.G. Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic. Ceram. Int. 2020, 46, 20576–20581.
- Ma, B.S.; Zhu, Y.; Wang, K.W.; Sun, Z.Z.; Ren, K.; Wang, Y.G. Reactive flash sintering and electrical transport properties of high-entropy (MgCoNiCuZn)1−xLixO oxides. J. Am. Ceram. Soc. 2022, 105, 3765–3773.
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer–derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837.
- Greil, P. Acive-filler-controlled pyrolysis of preceramic polymers. J. Am. Ceram. Soc. 1995, 78, 835–848.
- Bill, J.; Heimann, D. Polymer-derived ceramic coatings on C/C-SiC composites. J. Eur. Ceram. Soc. 1996, 16, 1115–1120.
- Schulz, M. Polymer derived ceramics in MEMS/NEMS—A review on production processes and application. Adv. Appl. Ceram. 2009, 108, 454–460.
- Jones, R.; Szweda, R.; Petrak, D. Polymer derived ceramic matrix composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 569–575.
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282.
- Liu, J.; Zhang, L.; Liu, Q.; Cheng, L.; Wang, Y.G. Polymer–derived SiOC–barium–strontium aluminosilicate coatings as an environmental barrier for C/SiC Composites. J. Am. Ceram. Soc. 2010, 93, 4148–4152.
- Wang, Y.G.; Liu, J. Corrosion of barium aluminosilicates by water–vapor: An investigation from first principles. Corros. Sci. 2009, 51, 2126–2129.
- More, K.L.; Tortorelli, P.F. Evaluating the Stability of BSAS–Based EBCs in High Water–Vapor Pressure Environments; Power for Land, Sea, & Air: Vienna, Austria, 2004; pp. 1–8.
- Haug, T.; Knale, H.; Ehrmann, U. Processing, properties and structure development of polymer–derived fiber–reinforced SiC. J. Am. Ceram. Soc. 1989, 72, 103–110.
- Nakano, K.; Kamiya, A.; Nishino, Y.; Imura, T.; Chou, T.W. Fabrication and characterization of three–dimensional carbon fiber reinforced silicon carbide and silicon nitride composites. J. Am. Ceram. Soc. 1995, 78, 2811–2814.
- Naslain, R. Design, preparation and properties of non–oxide CMCs for application in engines and nuclear reactors: An overview. Compos. Sci. Technol. 2004, 64, 155–170.
- Riedel, R.; Seher, M.; Mayer, J.; Szabó, D.V. Polymer–derived Si–based bulk ceramics, Part I: Preparation, processing and properties. J. Am. Ceram. Soc. 1995, 15, 703–715.
- Raju, K.; Yoon, D.H. Sintering additives for SiC based on the reactivity: A review. Ceram. Int. 2016, 42, 17947–17962.
- Kim, D.H.; Kim, C.H. Toughening behavior of silicon carbide with additions of yttria and alumina. J. Am. Ceram. Soc. 1990, 73, 1431–1434.
- Jang, C.W.; Kim, J.; Kang, S.L. Effect of sintering atmosphere on grain shape and grain growth in liquid-phase-sintered silicon carbide. J. Am. Ceram. Soc. 2002, 85, 1281–1284.
- Costello, J.A.; Tressler, R.E. Oxidation of silicon carbide crystals and ceramics: In dry oxygen. J. Am. Ceram. Soc. 1986, 69, 674–681.
- Singhal, C.; Lange, F.F. Effect of alumina content on the oxidation of hot–pressed silicon carbide. J. Am. Ceram. Soc. 1975, 58, 133–135.
- Wang, Y.G. Polymer–Derived Si-Al-C-N Ceramics: Oxidation, Hot–Corrosion and Structural Evolution. Ph.D Thesis, Dissertation of the University of Central Florida, Orlando, FL, USA, 2006.
- He, J.; Gao, Y.; Wang, Y.G.; Fang, J.; An, L. Synthesis of ZrB2-SiC nanocomposite powder via polymeric precursor route. Ceram. Int. 2017, 43, 1602–1607.
- He, J.; Cao, Y.; Zhang, Y.; Wang, Y.G. Mechanical properties of ZrB2–SiC ceramics prepared by polymeric precursor route. Ceram. Int. 2018, 44, 6520–6526.
- Raj, R.; An, L.; Shah, S.; Riedel, R.; Fasel, C.; Kleebe, H.J. Oxidation kinetics of an amorphous silicon carbonitride ceramic. J. Am. Ceram. Soc. 2001, 84, 1803–1810.
- Riedel, R.; Ruwisch, L.M.; An, L.; Raj, R. Amorphous silicoboron carbonitride ceramics with anomalously high resistance to creep. J. Am. Ceram. Soc. 1998, 81, 3341–3344.
- An, L.; Riedel, R.; Konetschny, C.; Kleebe, H.J.; Raj, R. Newtonian viscosity of amorphous silicon carbonitride at high temperature. J. Am. Ceram. Soc. 1998, 81, 1349–1352.
- Thum, G.; Canel, J.; Bill, J.; Aldinger, F. Compression creep behavior of precursor-derived Si–C–N ceramics. J. Eur. Ceram. Soc. 1999, 19, 2317–2323.
- Wang, Y.G.; Fan, Y.; Zhang, L.; Zhang, W.; An, L. Polymer–derived SiAlCN ceramics resist to oxidation at 1400 °C. Scr. Mater. 2006, 55, 295–297.
- Wang, Y.G.; Fei, W.; Fan, Y.; Zhang, L.; Zhang, W.; An, L. A silicoaluminum carbonitride ceramic resist oxidation/corrosion in water vapor. J. Mater. Res. 2006, 21, 1625–1628.
- An, L.; Wang, Y.G.; Bharadwaj, L.; Zhang, L.; Fan, Y.; Jiang, D.; Sohn, Y.; Desai, V.H.; Kapat, J.; Chow, L.C. Silicoaluminum carbonitride with anomalously high resistance to oxidation and hot corrosion. Adv. Eng. Mater. 2004, 6, 337–340.
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478.
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon–containing polymer–derived ceramic nanocomposites (PDC–NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052.
- Vakifahmetoglu, C. Fabrication and properties of ceramic 1D nanostructures from preceramic polymers: A review. Adv. Appl. Ceram. 2011, 110, 188–204.
- Li, H.; Zhang, L.; Cheng, L.; Yu, Z.; Huang, M.; Tu, H.; Xia, H. Effect of curing and pyrolysis processing on the ceramic yield of a highly branched polycarbosilane. J. Mater. Sci. 2009, 44, 721–725.
- Hauser, A.Q.; Honnef, K.; Hanemann, T. Crosslinking behavior of UV-cured polyorganosilazane as polymer-derived ceramic precursor in ambient and nitrogen atmosphere. Polymers 2021, 13, 2424.
- Zhang, L.G.; Wang, Y.S.; Wei, Y.; Xu, W.; Fang, D.; Zhai, L.; Lin, K.C.; An, L. A silicon carbonitride ceramic with anomalously high piezoresistivity. J. Am. Ceram. Soc. 2008, 91, 1346–1349.
- Liu, W.; Cao, Y.; Cheng, L.; Wang, Y. Design of polymer-derived SiC for nuclear applications from the perspective of heterogeneous interfaces. J. Eur. Ceram. Soc. 2018, 38, 469–478.
- Packirisamy, S.; Sreejith, K.J.; Devapal, D.; Swaminathan, B. Handbook of Advanced Ceramics and Composites: Polymer-Derived Ceramics and Their Space Applications; Springer Nature: Cham, Switzerland, 2020; pp. 999–1000.
- Gunji, T.; Sopyan, I.; Abe, Y. Synthesis of polytitanosiloxanes and their transformation to SiO2–TiO2 ceramic fibers. J. Polym. Sci. A Polym. Chem. 1994, 32, 3133–3313.
- Liu, C.; Pan, R.; Hong, C.; Zhang, X.; Han, W.; Han, J.; Du, S. Effects of Zr on the precursor architecture and high-temperature nanostructure evolution of SiOC polymer derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 395–402.
- Hotza, D.; Nishihora, R.K.; Machado, R.A.F.; Geffroy, P.M.; Chartier, T.; Bernard, S. Tape casting of preceramic polymers towards advanced ceramics: A review. Int. J. Ceram. Eng. Sci. 2019, 1, 21–41.
This entry is offline, you can click here to edit this entry!
