Hydrogen as an energy carrier is a promising alternative to fossil fuels, and it becomes more and more popular in developed countries as a carbon-free fuel. The low boiling temperature of hydrogen (20 K or −253.15 °C) provides a unique opportunity to implement superconductors with a critical temperature above 20 K such as MgB2 or high-temperature superconductors. Superconductors increase efficiency and reduce the loss of energy, which could compensate for the high price of LH2 to some extent. Norway is one of the pioneer countries with adequate infrastructure for using liquid hydrogen in the industry, especially in marine technology where a superconducting propulsion system can make a remarkable impact on its economy. Using superconductors in the motor of a propulsion system can increase its efficiency from 95% to 98% when the motor operates at full power. The difference in efficiency is even greater when the motor does not work at full power.
- superconductors
- liquid hydrogen
- hydrogen economy
- marine industry
- MgB2
1. Introduction
-
Hydrogen has a wide range of uses in transportation and industries [5];
2. Hydrogen Economy
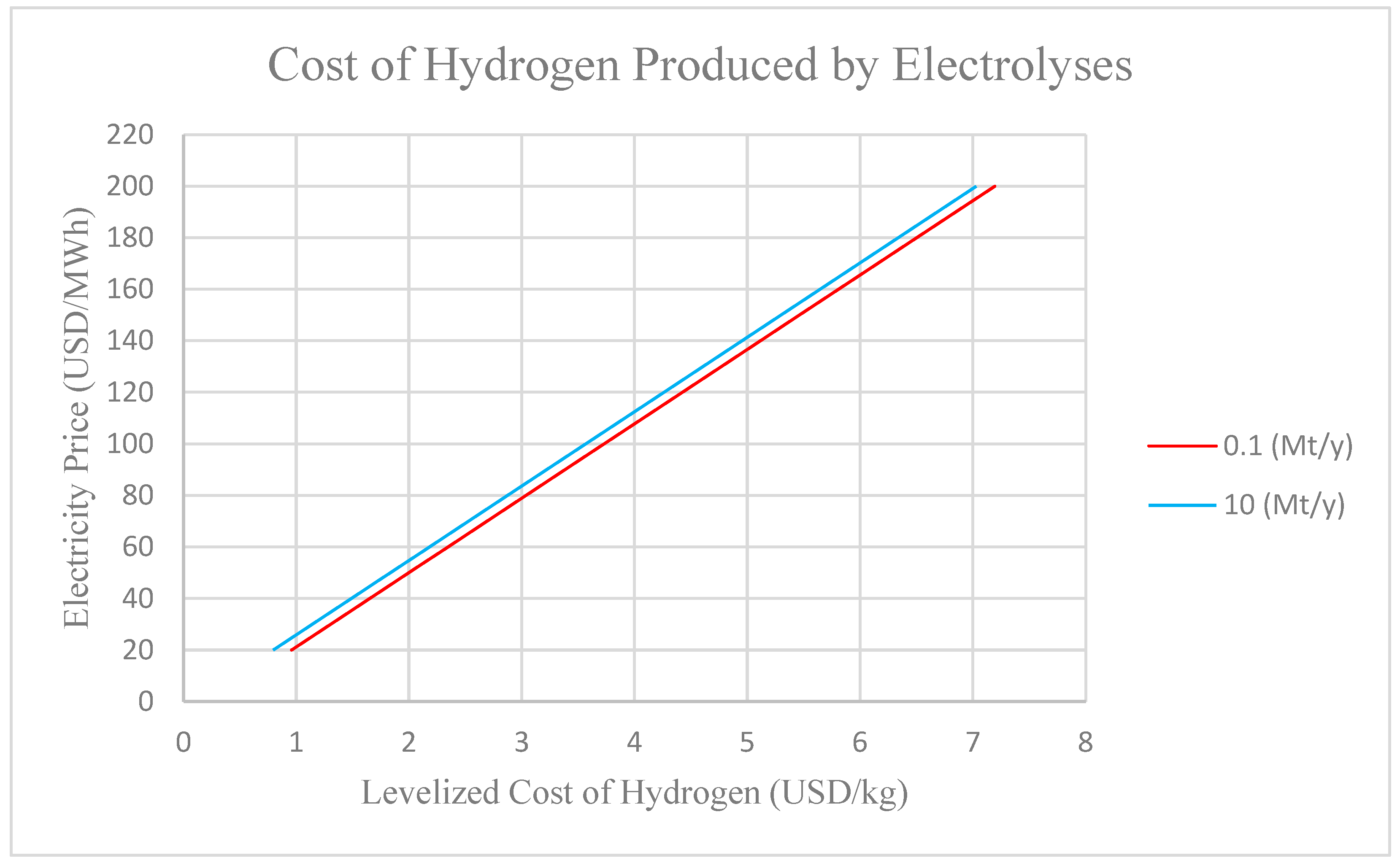
3. Superconductivity Applications
This entry is adapted from the peer-reviewed paper 10.3390/en15176138
References
- Gupta, R.; Basile, A.; Veziroglu, T.N. Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure; Woodhead Publishing: Swaston, UK, 2016.
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086.
- Damman, S.; Sandberg, E.; Rosenberg, E.; Pisciella, P.; Johansen, U. Largescale hydrogen production in Norway-possible transition pathways towards 2050. SINTEF Rapp. 2020. Available online: https://hdl.handle.net/11250/2649737 (accessed on 14 February 2020).
- Hamborg, E.S.; Gulbrandsen, T.H.; Steeneveldt, R.; Svenes, S.; Berggren, H.; Kvarsvik, S.; Pettersen, J. Norwegian Hydrogen Value Chain Demonstration Based on Decarbonized Natural Gas. In Proceedings of the 15th International Conference on Greenhouse Gas Control Technologies, Abu Dhabi, United Arab Emirates, 15–18 March 2021.
- Van de Graaf, T.; Overland, I.; Scholten, D.; Westphal, K. The new oil? The geopolitics and international governance of hydrogen. Energy Res. Soc. Sci. 2020, 70, 101667.
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85.
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843.
- Moreno-Benito, M.; Agnolucci, P.; Papageorgiou, L.G. Towards a sustainable hydrogen economy: Optimisation-based framework for hydrogen infrastructure development. Comput. Chem. Eng. 2017, 102, 110–127.
- Petrescu, R.V.V.; Machin, A.; Fontánez, K.; Arango, J.C.; Marquez, F.M.; Petrescu, F.I.T. Hydrogen for aircraft power and propulsion. Int. J. Hydrogen Energy 2020, 45, 20740–20764.
- Stroman, R.O.; Schuette, M.W.; Swider-Lyons, K.; Rodgers, J.A.; Edwards, D.J. Liquid hydrogen fuel system design and demonstration in a small long endurance air vehicle. Int. J. Hydrogen Energy 2014, 39, 11279–11290.
- Ball, M.; Weeda, M. The hydrogen economy–vision or reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919.
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668.
- Demirbas, A. Future hydrogen economy and policy. Energy Sources Part B Econ. Plan. Policy 2017, 12, 172–181.
- Norway’s NEL Targets $1.5/kg Green Hydrogen Cost by 2025. Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/electric-power/012121-norways-nel-targets-15kg-green-hydrogen-cost-by-2025 (accessed on 21 January 2021).
- Norwegian Future Value Chains for Liquid Hydrogen. Available online: https://maritimecleantech.no/wp-content/uploads/2016/11/Report-liquid-hydrogen.pdf (accessed on 21 August 2022).
- Meca, V.L.; d’Amore-Domenech, R.; Crucelaegui, A.; Leo, T.J. Large-Scale Maritime Transport of Hydrogen: Economic Comparison of Liquid Hydrogen and Methanol. ACS Sustain. Chem. Eng. 2022, 10, 4300–4311.
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrogen Energy 2017, 42, 13329–13338.
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044.
- Ishimoto, Y.; Voldsund, M.; Nekså, P.; Roussanaly, S.; Berstad, D.; Gardarsdottir, S.O. Large-scale production and transport of hydrogen from Norway to Europe and Japan: Value chain analysis and comparison of liquid hydrogen and ammonia as energy carriers. Int. J. Hydrogen Energy 2020, 45, 32865–32883.
- Mojarrad, M. Simulation of Hydrogen Tank Refuelling. Master’s Thesis, University of South-Eastern Norway, Notodden, Norway, May 2020.
- Paster, M.D.; Ahluwalia, R.K.; Berry, G.; Elgowainy, A.; Lasher, S.; McKenney, K.; Gardiner, M. Hydrogen storage technology options for fuel cell vehicles: Well-to-wheel costs, energy efficiencies, and greenhouse gas emissions. Int. J. Hydrogen Energy 2011, 36, 14534–14551.
- Reuβ, M.; Dimos, P.; Léon, A.; Grube, T.; Robinius, M.; Stolten, D. Hydrogen Road Transport Analysis in the Energy System: A Case Study for Germany through 2050. Energies 2021, 14, 3166.
- Nerheim, A.R.; Æsøy, V.; Holmeset, F.T. Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems? J. Mar. Sci. Eng. 2021, 9, 743.
- Rosenberg, E.; Fidje, A.; Espegren, K.A.; Stiller, C.; Svensson, A.M.; Møller-Holst, S. Market penetration analysis of hydrogen vehicles in Norwegian passenger transport towards 2050. Int. J. Hydrogen Energy 2010, 35, 7267–7279.
- Brandon, N.; Kurban, Z. Clean energy and the hydrogen economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160400.
- Durrell, J.H.; Ainslie, M.D.; Zhou, D.; Vanderbemden, P.; Bradshaw, T.; Speller, S.; Filipenko, M.; Cardwell, D.A. Bulk superconductors: A roadmap to applications. Supercond. Sci. Technol. 2018, 31, 103501.
- Kitazawa, K. Superconductivity: 100th anniversary of its discovery and its future. Jpn. J. Appl. Phys. 2011, 51, 010001.
- Nishijima, S.; Eckroad, S.; Marian, A.; Choi, K.; Kim, W.S.; Terai, M.; Deng, Z.; Zheng, J.; Wang, J.; Umemoto, K.; et al. Superconductivity and the environment: A roadmap. Supercond. Sci. Technol. 2013, 26, 113001.
- Tomsic, M.; Rindfleisch, M.; Yue, J.; McFadden, K.; Phillips, J.; Sumption, M.D.; Bhatia, M.; Bohnenstiehl, S.; Collings, E.W. Overview of MgB2 superconductor applications. Int. J. Appl. Ceram. Technol. 2007, 4, 250–259.
- Plakida, N. High-Temperature Cuprate Superconductors: Experiment, Theory, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010.
- Baik, S.; Kwon, Y.; Park, S.; Kim, H. Performance analysis of a superconducting motor for higher efficiency design. IEEE Trans. Appl. Supercond. 2013, 23, 5202004.
- Terao, Y.; Seta, A.; Ohsaki, H.; Oyori, H.; Morioka, N. Lightweight design of fully superconducting motors for electrical aircraft propulsion systems. IEEE Trans. Appl. Supercond. 2019, 29, 1–5.
- Mikheenko, P. Superconductivity for hydrogen economy. J. Phys. Conf. Ser. 2011, 286, 012014.
- Terao, Y.; Sekino, M.; Ohsaki, H. Comparison of conventional and superconducting generator concepts for offshore wind turbines. IEEE Trans. Appl. Supercond. 2012, 23, 5200904.
- Abrahamsen, A.B.; Mijatovic, N.; Seiler, E.; Zirngibl, T.; Træholt, C.; Nørgård, P.B.; Pedersen, N.F.; Andersen, N.H.; Østergaard, J. Superconducting wind turbine generators. Supercond. Sci. Technol. 2010, 23, 034019.
- Qu, R.; Liu, Y.; Wang, J. Review of superconducting generator topologies for direct-drive wind turbines. IEEE Trans. Appl. Supercond. 2013, 23, 5201108.
- Furuse, M.; Fuchino, S.; Okano, M.; Natori, N.; Yamasaki, H. Development of a cooling system for superconducting wind turbine generator. Cryogenics 2016, 80, 199–203.
- Del-Valle, N.; Sanchez, A.; Navau, C.; Chen, D.X. Magnet guideways for superconducting maglevs: Comparison between Halbach-type and conventional arrangements of permanent magnets. J. Low Temp. Phys. 2011, 162, 62–71.
- Werfel, F.N.; Floegel-Delor, U.; Rothfeld, R.; Riedel, T.; Goebel, B.; Wippich, D.; Schirrmeister, P. Superconductor bearings, flywheels and transportation. Supercond. Sci. Technol. 2011, 25, 014007.
- Berger, A.; Cherevatskiy, S.; Noe, M.; Leibfried, T. Comparison of the efficiency of superconducting and conventional transformers. J. Phys. Conf. Ser. 2010, 234, 032004.
- Dai, S.; Ma, T.; Qiu, Q.; Zhu, Z.; Teng, Y.; Hu, L. Development of a 1250-kVA superconducting transformer and its demonstration at the superconducting substation. IEEE Trans. Appl. Supercond. 2015, 26, 1–7.
- Glasson, N.; Staines, M.; Buckley, R.; Pannu, M.; Kalsi, S. Development of a 1 MVA 3-phase superconducting transformer using YBCO Roebel cable. IEEE Trans. Appl. Supercond. 2010, 21, 1393–1396.
- Vysotsky, V.S.; Fetisov, S.S.; Zubko, V.V.; Zanegin, S.Y.; Nosov, A.A.; Ryabov, S.M.; Bykovsky, N.V.; Svalov, G.G.; Volkov, E.P.; Fleishman, L.S.; et al. Development and test results of HTS windings for superconducting transformer with 1 MVA rated power. IEEE Trans. Appl. Supercond. 2016, 27, 1–5.
- Baig, T.; Al Amin, A.; Deissler, R.J.; Sabri, L.; Poole, C.; Brown, R.W.; Tomsic, M.; Doll, D.; Rindfleisch, M.; Peng, X.; et al. Conceptual designs of conduction cooled MgB2 magnets for 1.5 and 3.0 T full body MRI systems. Supercond. Sci. Technol. 2017, 30, 043002.
- Iwasa, Y. Towards liquid-helium-free, persistent-mode MgB2 MRI magnets: FBML experience. Supercond. Sci. Technol. 2017, 30, 053001.
- Parizh, M.; Lvovsky, Y.; Sumption, M. Conductors for commercial MRI magnets beyond NbTi: Requirements and challenges. Supercond. Sci. Technol. 2016, 30, 014007.
