Prokaryotic translation is the process by which messenger RNA is translated into proteins in prokaryotes.
- prokaryotes
- translation
1. Initiation
Initiation of translation in prokaryotes involves the assembly of the components of the translation system, which are: the two ribosomal subunits (50S and 30S subunits); the mature mRNA to be translated; the tRNA charged with N-formylmethionine (the first amino acid in the nascent peptide); guanosine triphosphate (GTP) as a source of energy, and the three prokaryotic initiation factors IF1, IF2, and IF3, which help the assembly of the initiation complex. Variations in the mechanism can be anticipated.
The ribosome has three active sites: the A site, the P site, and the E site. The A site is the point of entry for the aminoacyl tRNA (except for the first aminoacyl tRNA, which enters at the P site). The P site is where the peptidyl tRNA is formed in the ribosome. And the E site which is the exit site of the now uncharged tRNA after it gives its amino acid to the growing peptide chain.
The selection of an initiation site (usually an AUG codon) depends on the interaction between the 30S subunit and the mRNA template. The 30S subunit binds to the mRNA template at a purine-rich region (the Shine-Dalgarno sequence) upstream of the AUG initiation codon. The Shine-Dalgarno sequence is complementary to a pyrimidine rich region on the 16S rRNA component of the 30S subunit. This sequence has been evolutionarily conserved and plays a major role in the microbial world we know today. During the formation of the initiation complex, these complementary nucleotide sequences pair to form a double stranded RNA structure that binds the mRNA to the ribosome in such a way that the initiation codon is placed at the P site.
Well-known coding regions that do not have AUG initiation codons are those of lacI (GUG)[1] and lacA (UUG) in the E. coli lac operon.[2] Two studies have independently shown that 17 or more non-AUG start codons may initiate translation in E. coli.[3][4]
2. Elongation
Elongation of the polypeptide chain involves addition of amino acids to the carboxyl end of the growing chain. The growing protein exits the ribosome through the polypeptide exit tunnel in the large subunit.[5]
Elongation starts when the fMet-tRNA enters the P site, causing a conformational change which opens the A site for the new aminoacyl-tRNA to bind. This binding is facilitated by elongation factor-Tu (EF-Tu), a small GTPase. For fast and accurate recognition of the appropriate tRNA, the ribosome utilizes large conformational changes (conformational proofreading).[6] Now the P site contains the beginning of the peptide chain of the protein to be encoded and the A site has the next amino acid to be added to the peptide chain. The growing polypeptide connected to the tRNA in the P site is detached from the tRNA in the P site and a peptide bond is formed between the last amino acids of the polypeptide and the amino acid still attached to the tRNA in the A site. This process, known as peptide bond formation, is catalyzed by a ribozyme (the 23S ribosomal RNA in the 50S ribosomal subunit). Now, the A site has the newly formed peptide, while the P site has an uncharged tRNA (tRNA with no amino acids). The newly formed peptide in the A site tRNA is known as dipeptide and the whole assembly is called dipeptidyl-tRNA. The tRNA in the P site minus the amino acid is known to be deacylated. In the final stage of elongation, called translocation, the deacylated tRNA (in the P site) and the dipeptidyl-tRNA (in the A site) along with its corresponding codons move to the E and P sites, respectively, and a new codon moves into the A site. This process is catalyzed by elongation factor G (EF-G). The deacylated tRNA at the E site is released from the ribosome during the next A-site occupation by an aminoacyl-tRNA again facilitated by EF-Tu.[7]
The ribosome continues to translate the remaining codons on the mRNA as more aminoacyl-tRNA bind to the A site, until the ribosome reaches a stop codon on mRNA(UAA, UGA, or UAG).
The translation machinery works relatively slowly compared to the enzyme systems that catalyze DNA replication. Proteins in prokaryotes are synthesized at a rate of only 18 amino acid residues per second, whereas bacterial replisomes synthesize DNA at a rate of 1000 nucleotides per second. This difference in rate reflects, in part, the difference between polymerizing four types of nucleotides to make nucleic acids and polymerizing 20 types of amino acids to make proteins. Testing and rejecting incorrect aminoacyl-tRNA molecules takes time and slows protein synthesis. In bacteria, translation initiation occurs as soon as the 5' end of an mRNA is synthesized, and translation and transcription are coupled. This is not possible in eukaryotes because transcription and translation are carried out in separate compartments of the cell (the nucleus and cytoplasm).
3. Termination
Termination occurs when one of the three termination codons moves into the A site. These codons are not recognized by any tRNAs. Instead, they are recognized by proteins called release factors, namely RF1 (recognizing the UAA and UAG stop codons) or RF2 (recognizing the UAA and UGA stop codons). These factors trigger the hydrolysis of the ester bond in peptidyl-tRNA and the release of the newly synthesized protein from the ribosome. A third release factor RF-3 catalyzes the release of RF-1 and RF-2 at the end of the termination process.
4. Recycling
The post-termination complex formed by the end of the termination step consists of mRNA with the termination codon at the A-site, an uncharged tRNA in the P site, and the intact 70S ribosome. Ribosome recycling step is responsible for the disassembly of the post-termination ribosomal complex.[8] Once the nascent protein is released in termination, Ribosome Recycling Factor and Elongation Factor G (EF-G) function to release mRNA and tRNAs from ribosomes and dissociate the 70S ribosome into the 30S and 50S subunits. IF3 then replaces the deacylated tRNA releasing the mRNA. All translational components are now free for additional rounds of translation.
5. Polysomes
Translation is carried out by more than one ribosome simultaneously. Because of the relatively large size of ribosomes, they can only attach to sites on mRNA 35 nucleotides apart. The complex of one mRNA and a number of ribosomes is called a polysome or polyribosome.[9]
6. Regulation of Translation
When bacterial cells run out of nutrients, they enter stationary phase and downregulate protein synthesis. Several processes mediate this transition.[10] For instance, in E. coli, 70S ribosomes form 90S dimers upon binding with a small 6.5 kDa protein, ribosome modulation factor RMF.[11][12] These intermediate ribosome dimers can subsequently bind a hibernation promotion factor (the 10.8 kDa protein, HPF) molecule to form a mature 100S ribosomal particle, in which the dimerization interface is made by the two 30S subunits of the two participating ribosomes.[13] The ribosome dimers represent a hibernation state and are translationally inactive.[14] A third protein that can bind to ribosomes when E. coli cells enter the stationary phase is YfiA (previously known as RaiA).[15] HPF and YfiA are structurally similar, and both proteins can bind to the catalytic A- and P-sites of the ribosome.[16][17] RMF blocks ribosome binding to mRNA by preventing interaction of the messenger with 16S rRNA.[18] When bound to the ribosomes the C-terminal tail of E. coli YfiA interferes with the binding of RMF, thus preventing dimerization and resulting in the formation of translationally inactive monomeric 70S ribosomes.[18][19]
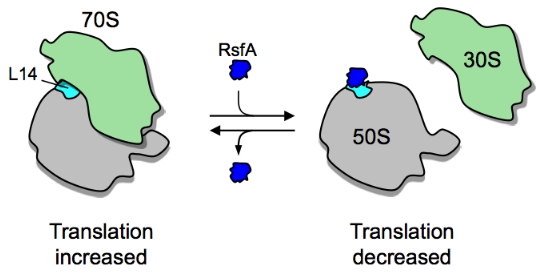
In addition to ribosome dimerization, the joining of the two ribosomal subunits can be blocked by RsfS (formerly called RsfA or YbeB).[20] RsfS binds to L14, a protein of the large ribosomal subunit, and thereby blocks joining of the small subunit to form a functional 70S ribosome, slowing down or blocking translation entirely. RsfS proteins are found in almost all eubacteria (but not archaea) and homologs are present in mitochondria and chloroplasts (where they are called C7orf30 and iojap, respectively). However, it is not known yet how the expression or activity of RsfS is regulated.
Another ribosome-dissociation factor in Escherichia coli is HflX, previously a GTPase of unknown function. Zhang et al. (2015) showed that HflX is a heat shock–induced ribosome-splitting factor capable of dissociating vacant as well as mRNA-associated ribosomes. The N-terminal effector domain of HflX binds to the peptidyl transferase center in a strikingly similar manner as that of the class I release factors and induces dramatic conformational changes in central intersubunit bridges, thus promoting subunit dissociation. Accordingly, loss of HflX results in an increase in stalled ribosomes upon heat shock and possibly other stress conditions.[21]
7. Effect of Antibiotics
Several antibiotics exert their action by targeting the translation process in bacteria. They exploit the differences between prokaryotic and eukaryotic translation mechanisms to selectively inhibit protein synthesis in bacteria without affecting the host.
The content is sourced from: https://handwiki.org/wiki/Biology:Prokaryotic_translation
References
- "Sequence of the lacI gene". Nature 274 (5673): 765–9. August 1978. doi:10.1038/274765a0. PMID 355891. https://dx.doi.org/10.1038%2F274765a0
- E.coli lactose operon with lacI, lacZ, lacY and lacA genes - Nucleotide - NCBI. 1993-05-05. https://www.ncbi.nlm.nih.gov/nuccore/146575?itemID=6&report=gbwithparts#feature_146575. Retrieved 2017-03-01.
- "Measurements of translation initiation from all 64 codons in E. coli". Nucleic Acids Research 45 (7): 3615–3626. April 2017. doi:10.1093/nar/gkx070. PMID 28334756. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5397182
- "A Comprehensive, High-Resolution Map of a Gene's Fitness Landscape". Molecular Biology and Evolution 33 (5): 1581–1592. May 2016. doi:10.1093/molbev/msu081. PMID 26912810. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4839222
- Structure of the E. coli protein-conducting channel bound to at translating ribosome, K. Mitra, et al. Nature (2005), vol 438, p 318
- "The ribosome as an optimal decoder: a lesson in molecular recognition". Cell 153 (2): 471–9. April 2013. doi:10.1016/j.cell.2013.03.032. PMID 23582332. https://dx.doi.org/10.1016%2Fj.cell.2013.03.032
- "Deacylated tRNA is released from the E site upon A site occupation but before GTP is hydrolyzed by EF-Tu". Nucleic Acids Research 33 (16): 5291–6. 2005. doi:10.1093/nar/gki833. PMID 16166657. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1216338
- "The ribosome-recycling step: consensus or controversy?". Trends in Biochemical 31 (3): 143–9. March 2006. doi:10.1016/j.tibs.2006.01.007. PMID 16487710. https://jdc.jefferson.edu/cgi/viewcontent.cgi?article=1005&context=bmpfp.
- Alberts, Bruce (2017). Molecular Biology of the Cell (6th ed.). Garland Science. pp. 301–303.
- "Lactococcus lactis YfiA is necessary and sufficient for ribosome dimerization". Molecular Microbiology 91 (2): 394–407. January 2014. doi:10.1111/mmi.12468. PMID 24279750. https://dx.doi.org/10.1111%2Fmmi.12468
- "Regulation of the Escherichia coli rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control". The EMBO Journal 12 (2): 625–30. February 1993. doi:10.1002/j.1460-2075.1993.tb05695.x. PMID 8440252. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=413246
- "Escherichia coli ribosome-associated protein SRA, whose copy number increases during stationary phase". Journal of Bacteriology 183 (9): 2765–73. May 2001. doi:10.1128/JB.183.9.2765-2773.2001. PMID 11292794. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=99491
- "Structure of the 100S ribosome in the hibernation stage revealed by electron cryomicroscopy". Structure 18 (6): 719–24. June 2010. doi:10.1016/j.str.2010.02.017. PMID 20541509. https://dx.doi.org/10.1016%2Fj.str.2010.02.017
- "Ribosome modulation factor: stationary growth phase-specific inhibitor of ribosome functions from Escherichia coli". Biochemical and Biophysical Research Communications 214 (2): 410–7. September 1995. doi:10.1006/bbrc.1995.2302. PMID 7677746. https://dx.doi.org/10.1006%2Fbbrc.1995.2302
- "A protein residing at the subunit interface of the bacterial ribosome". Proceedings of the National Academy of Sciences of the United States of America 96 (22): 12345–9. October 1999. doi:10.1073/pnas.96.22.12345. PMID 10535924. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=22919
- "Structural basis for the control of translation initiation during stress". Nature Structural & Molecular Biology 11 (11): 1054–9. November 2004. doi:10.1038/nsmb850. PMID 15502846. https://dx.doi.org/10.1038%2Fnsmb850
- "Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ". The Journal of Cell Biology 190 (4): 613–21. August 2010. doi:10.1083/jcb.201005007. PMID 20733057. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2928015
- "How hibernation factors RMF, HPF, and YfiA turn off protein synthesis". Science 336 (6083): 915–8. May 2012. doi:10.1126/science.1218538. PMID 22605777. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3377384
- "Ribosome binding proteins YhbH and YfiA have opposite functions during 100S formation in the stationary phase of Escherichia coli". Genes to Cells 10 (12): 1103–12. December 2005. doi:10.1111/j.1365-2443.2005.00903.x. PMID 16324148. https://dx.doi.org/10.1111%2Fj.1365-2443.2005.00903.x
- "RsfA (YbeB) proteins are conserved ribosomal silencing factors". PLoS Genetics 8 (7): e1002815. 2012. doi:10.1371/journal.pgen.1002815. PMID 22829778. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3400551
- "HflX is a ribosome-splitting factor rescuing stalled ribosomes under stress conditions". Nature Structural & Molecular Biology 22 (11): 906–13. November 2015. doi:10.1038/nsmb.3103. PMID 26458047. https://dx.doi.org/10.1038%2Fnsmb.3103
