1. Metal Doping
Noble metals such as Au, Ag, Pd, or Pt as well as transition metals such as V, Cr, Fe, Co, Cu, Mo, Ni, and Zn were used to dope titania and improve its absorption of visible light [
40,
47,
61,
62,
63,
64,
65,
66]. Doping with noble metals can both enable absorption of longer wavelengths and improve charge carrier separation [
67,
68,
69]. This is achieved by the circumstance that the Fermi levels of the doped metals are localized below the conduction band of the TiO
2. By metal doping, Schottky barriers can form at the TiO
2-metal interface, which behave like electron traps. The photoexcited electrons migrate from the TiO
2 to the metal through the Schottky barrier until the Fermi levels are equalized, while the photogenerated holes remain in the TiO
2. This effect improves charge separation and results in a lower electron-hole recombination [
70,
71].
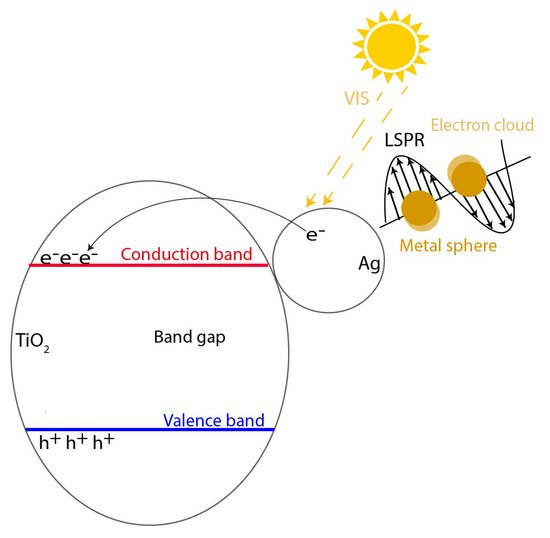
The absorption of longer wavelengths due to noble metal doping is usually associated with the effect of surface plasmon resonance. Surface plasmons are caused by a collective oscillation of free charge carriers on the surface of conducting materials upon interaction with light (
Figure 1). Resonance occurs when the particles are irradiated with light corresponding to their plasmon frequency, resulting in induced electric fields on the surface of the particles. Regarding the generated electric fields, it was shown that the generated electric fields can lead to a one thousand times larger charge carrier formation compared to the incident electric field. The plasmon frequency can be highly influenced and depends on factors such as nanoparticle material, size, and shape [
72].
Figure 1. Schematic representation of the surface plasmon resonance (SPR) effect of silver doped TiO2.
The positive influence of noble metal doping on charge separation in titania was proven, e.g., in the removal of
Mycobacterium tuberculosis. Here, HEPA filter were coated with silver-doped TiO
2. Due to the doping, a complete removal of
M. tuberculosis was achieved after only 100 min, while undoped TiO
2 required about 180 min for a complete removal. A concentration dependence was also observed, suggesting that an excessively high concentration of Ag leads to increased recombination, since Ag becomes the recombination center [
62].
The absorption of longer wavelengths due to the surface plasmon resonance effect was shown by doping of TiO
2 with Au. Here, enhanced absorption was measured at wavelengths of about 500 nm. Results from emission photoluminescence spectra indicated that additional energy levels are present at 2.7 eV and 2.54 eV, which were assigned to oxygen vacancy states. In addition, emission photoluminescence spectra results refer to a lower recombination of the Au doped samples compared to pure TiO
2. Both effects led to higher photoactivity in the degradation of Rhodamine B under simulated solar light for Au doped TiO
2 as compared to the undoped sample [
47].
By doping TiO
2 with Pd, the band gap could be decreased from 3.2 eV for pure TiO
2 to 3.07 eV for the highest Pd concentration. It was observed that with increasing Pd concentration the band gap got smaller. Doping with Pd at various concentrations resulted in higher photoactivity under UV irradiation compared to pure TiO
2. Regarding the concentration, it was measured that the photoactivity increases up to a limiting concentration and finally decreases with increasing concentration. It was concluded that excess doping leads to a conglomeration of Pd particles, which then act as recombination centers [
40].
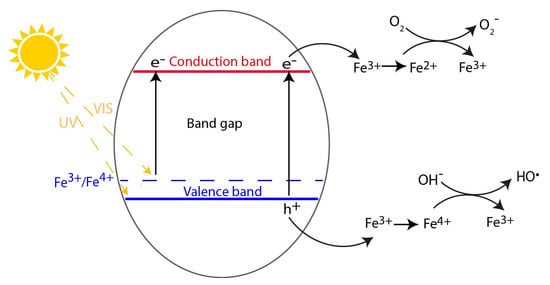
Doping with transition metals can also positively influence the photoactivity of TiO
2. Doping with Fe results in an Fe
3+/Fe
4+ energy level above the valence band of TiO
2 and an Fe
3+/Fe
2+ energy level located below the conduction band (
Figure 2). This allows the material to absorb photons at wavelengths > 400 nm. Electrons can be photoexcited from the Fe
3+ interband state (which generates Fe
4+) to the conduction band of TiO
2. Fe
3+ can serve both as hole or electron trap, since both resulting oxidation states of Fe
4+ and Fe
2+ can be formed. According to crystal field theory, Fe
2+ and Fe
4+ are relatively unstable as compared to Fe
3+, which has half-filled 3d orbitals. Therefore, charge transfer from Fe
4+ and Fe
2+, respectively, to reaction species in air or water is preferred (
Figure 2). Thus, doping with Fe
3+ can cause a better (and faster) charge separation. However, Fe
3+ can also become a recombination center, if the concentration of the ions is too high, leading to a decrease in photoactivity [
73,
74]. The influence of Fe doping on the photocatalytic properties of P25 was studied upon the decomposition of acetaldehyde. It was found that the band gap decreased with increasing Fe content and was only 2.3 eV for an Fe content of 1.8 wt%. Under visible light irradiation, up to 65% of acetaldehyde could be removed after 1050 min for the Fe doped samples, while pure TiO
2 could only remove 40% of acetaldehyde. It is worth mentioning that the highest decomposition rate was observed for the lowest Fe concentration and the photoactivity declined with increasing Fe concentration. It was concluded that the higher Fe content increased the recombination, which resulted in lower photoactivity [
63].
Figure 2. Energy diagram for doping with iron and possible consecutive reaction mechanisms under UV and visible light irradiation.
Also Cu and Mn doping was studied. When titania thin films are doped with Mn or Cu, an improvement in photocatalytic properties was observed with Mn doping, while a decline was observed for Cu doping. Since the photocatalytic properties depended on the Mn and Cu concentration, it was assumed that the Cu concentration was already too high for an improved activity. At low concentrations, the metals seem to contribute to lower recombination, while at high concentration they become recombination centers. Doping with Mn also improved the antibacterial properties compared to pure TiO
2 [
64].
2. Non-Metal Doping
Doping with non-metals has mainly been carried out with N, S, C, and B [
75,
76,
77,
78,
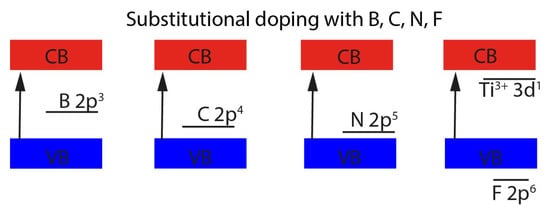
79]. The resulting properties vary depending on whether the non-metals are substitutional for oxygen or are incorporated as interstitial atoms. It is assumed that the substitution of oxygen mainly affects the valence band, which is built of O 2p states, yet there seem to be exceptions such as doping with fluorine. Here, the excess electron is lifted into the empty titanium levels and trapped in the Ti 3d orbital, which lies below the conduction band. Doping with lighter elements than oxygen such as N, C, and B causes the valence band to be depleted by one to three electrons depending on which element was used. It can be seen that the lower the atomic number, the higher the energy of the 2p levels.
Figure 3 shows that the energy of the 2p states is increasing in the order of N, C, and B. The B 2p state has the highest energy level which leads to a smaller band gap [
75].
Figure 3. Schematic representation of the energy levels for substitutional doping in TiO2.
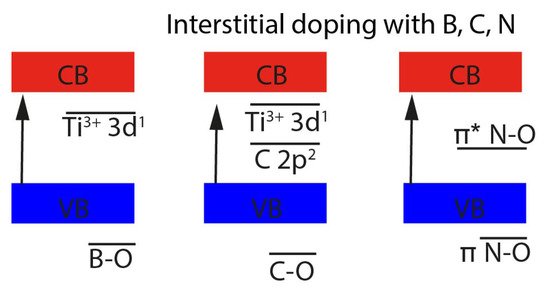
In contrast, the very small non-metal atoms can also diffuse through the interstitial spaces and bind to interstitial atoms. Depending on the redox potential of the doped element, they can be oxidized. For example, B gives up all three valence electrons to Ti interstitial ions, which is why three Ti
3+ ions are formed. The boron atom is now bound to either 3 or 4 oxygen atoms, the three new B-O levels are below the valence band while the Ti
3+ levels are below the conduction band [
75,
76] (
Figure 4). The interstitial carbon atom has a higher electronegativity compared to B, which is why C only gives up two of the four valence electrons. This leads to the formation of two Ti
3+ species, which are located below the conduction band. The levels of the three C-O bonds are below the valence band, while the additional C 2p pair is located in the band gap [
75,
76]. Nitrogen as a doping element is found to preferentially bind only one interstitial oxygen. The N atom pushes the oxygen atoms out of the Ti-O-Ti plane towards the interstitial space, resulting in an NO species with two additional electrons. Thus, it binds to the interstitial Ti with the
π bonding system. As shown in
Figure 4, the
π NO states are below the valence band, while the
π* NO states are within the band gap [
75,
76]. In order to study the influence of N, single wall TiO
2-carbon nanotubes (SWCNT) were doped with N. As a result, the methyl orange degradation under visible light was increased from about 40% for pure TiO
2 to about 72% for the doped material. Without the SWCNT as substrates, the degradation was at 50% [
77].
Figure 4. Schematic representation of the energy levels for interstitial doping in TiO2.
The co-doping of N and F resulted in a higher activity for the methylene blue degradation under visible light than for the doping with N only. With increasing F content, an additionally improved photoactivity could be measured. From the results of XRD and absorption spectra, it was concluded that doping with F prevents the growth of TiO
2 particles and enhances the absorption in the visible range [
79].
Fan et al. doped TiO2 with C which shifted the onset of the absorption curve of the doped sample to about 700 nm. The doped TiO2 was able to degrade 60% of the methyl orange (λ > 400 nm) in a test after half an hour, while the undoped TiO2 showed negligible degradation.
Our literature survey exhibited that doping and co-doping techniques are increasingly used in real applications. The doping with noble metals is obviously in many cases too expensive to manufacture coatings or admixtures on large scales and is hence rarely applied. In contrast, non-metals such as carbon are much more suitable. One interesting application of a carbon-doped titania is asphalt coating. The doped sample showed a complete degradation of NO
x in a confined environment after 90 min. For undoped TiO
2, the NO
x concentration was still at about 70% after 90 min. The sample was irradiated with a solar spectrum lamp. It was observed that the photoactivity also increased with increasing dopant concentration [
78]. More applications are to be discussed in the respective sections below.
This entry is adapted from the peer-reviewed paper 10.3390/inorganics10090139