Owing to their combinations of unique properties of sintered ceramics and the distinctive characteristics of glasses, glass-ceramics have attracted great interest from both academia and industry worldwide since their accidental discovery in 1953.
- glass-ceramics
- application
- properties
1. Introduction
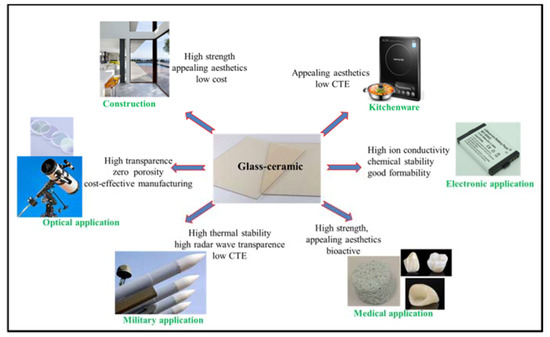
To date, glass-ceramics have been widely used in a wide range of fields in our daily life. The entry introduces the applications of glass-ceramics in five different fields, including construction, optical, military, biomedical, electronic and kitchenware. Meanwhile, the properties of glass-ceramics that enable them to be used in the above fields were also described accordingly.
2. Properties and Applications
Figure 1 demonstrates some of the applications of glass–ceramics.
2. 1. Construction Application
In the last few decades, there has been considerable research on the production of glass-ceramics from silicate waste, such as coal combustion ash, slag from steel production, fly ash and filter dusts from waste incinerators, mud from metal hydrometallurgy, etc[1][2]. These low-cost glass-ceramics recycled from industry silicate waste are generally strong, hard and chemically resistant. Their intended use is for abrasion and chemically resistant parts or floor and wall tile. One of the most popular glass–ceramic used in construction is Neopariés LT, with wollastonite as the main crystalline phase. Neopariés glass–ceramic panels are an ideal alternative to stone for interior and exterior applications.
2.2. Optical Application
In optical field, many glass–ceramics show high translucency or even can be transparent because of the fact that zero porosity can be relatively easily achieved. These make glass–ceramics excellent material for optical applications. For instance, transparent and low thermal expansion glass–ceramics based on lithium aluminosilicate (LAS) system have been used as telescope mirror blanks and laser gyroscopes [3].Glass-ceramics can demonstrate optical properties similar to those of single crystals, meanwhile, glass-ceramics tend to be more cost efficient and more suitable for manufacturing larger objects with intricate shapes. Moreover, glass-ceramics doped with transition metal ions were developed for use in broadband optical amplification, tunable and infrared lasers, phosphor with tunable UV/blue luminescence behavior, and in solar collectors[4][5].
2. 3. Military Application
In military field, glass–ceramics now are used in nosecones of high–performance aircraft and missiles. Materials used in these applications must exhibit a challenging combination of properties to withstand critical conditions resulting from high–speed flying in the atmosphere: Low coefficient of thermal expansion; high mechanical strength; high abrasion resistance; high radar wave transparency for navigation[6][7]. However, little has been published and patented on the chemical composition, microstructure, and preparation processes of the glass-ceramics used for military, because of the sensitive nature of this military-related research. No glass, metal or single crystal can simultaneously meet all of these relevant specifications, while glass–ceramics with the tailored properties were able to achieve the challenge. Another example is the view-window for armored vehicles or tank, which requires that material of the view-window to be both strong and highly-translucent. Glass-ceramics with high strength, high toughness, and high translucency are excellent candidates for these particular uses.
2.4. Medical Application
In medical field, bioglass, for instance the “gold standard” bioglass 45S5 invented by Larry Hench[8], has been successfully used in medical field. However, the inherent low strength and low toughness limit the application of bioglass as load-bearing biomaterial. With crystalline phases as strengthening and toughening phases, glass–ceramics overcome the weakness of bioglass. Moreover, glass-ceramics generally show better bioactivity and biocompability than sintered ceramics due to the presence of glass phase in glass-ceramics.For instance, A–W glass–ceramic that contains apatite and β–wollastonite (CaO·SiO2) crystals (with the commercial brand name of Cerabone) is considered as the most outstanding bioactive glass–ceramics for hard tissue repair[9].Research on improving the properties of glass-ceramic biomaterials are still going on and one direction is to introduce greater multifunctionality to glass-ceramics through inorganic modifications, for instance, bioactivity and antimicrobial activity can be simultaneously enhanced by incorporation of specific ions, such as Cu2+, Sr2+, and Zn2+ ions[10].
2.5. Electronic Application
In electronic field, all–solid–state secondary batteries with inorganic solid electrolytes are expected to be next–generation high–output batteries. The crucial requirements for appropriate solid electrolytes are high ionic conductivity and good formability. Different types of inorganic solid electrolytes made by glass–ceramics have been developed, for instance, glass–ceramics has the crystalline form of Li1+x+yAlxTi2−xSiyP3−yO12 exhibited a high lithium–ion conductivity of 10−3 S·cm−1[11]. Compared with liquid electrolytes, solid electrolytes made by glass-ceramics is more stable in the open atmosphere and even to exposure to moist air, thus, they are expected to be applied for various uses[12].
2.6. Kitchenware Application
In kitchenware field, higher toughness (compared with glass), appealing aesthetics, and very low thermal expansion coefficient make glass–ceramics the excellent material for kitchenware, such as cooktops, cookware, and bakeware. In the late 1960s, Corning introduced the concept of cooking on a smooth white glass-ceramic. To date, many glass-ceramics with different compositions have been developed to used as kitchenwares. The most widely used system is the Li2O–Al2O3–SiO2 (LAS) system with additional components, such as CaO, MgO, ZnO[7]. The main crystalline phase is a β–quartz solid solution, which has an overall negative CTE. LAS glass–ceramics can sustain repeated and quick temperature changes of 800 to 1000 °C[13].
This entry is adapted from the peer-reviewed paper 10.3390/ma13051049
References
- Ning Xie; Jonathan. L. Bell; Waltraud Kriven; Fabrication of Structural Leucite Glass-Ceramics from Potassium-Based Geopolymer Precursors. Journal of the American Ceramic Society 2010, 93, 2644-2649, 10.1111/j.1551-2916.2010.03794.x.
- Wolfgang Wisniewski; Katrin Thieme; Christian Rüssel; Fresnoite glass-ceramics – A review. Progress in Materials Science 2018, 98, 68-107, 10.1016/j.pmatsci.2018.05.002.
- I. Alekseeva; Olga Dymshits; M. Tsenter; Alexandr Zhilin; V. Golubkov; I. Denisov; N. Skoptsov; A. Malyarevich; Konstantin Yumashev; Optical applications of glass-ceramics. Journal of Non-Crystalline Solids 2010, 356, 3042-3058, 10.1016/j.jnoncrysol.2010.05.103.
- V. Fuertes; José F. Fernández; Esther Enríquez; Tunable UV/blue luminescence in rare-earth free glass-ceramic phosphor. Journal of the European Ceramic Society 2019, 39, 3221-3228, 10.1016/j.jeurceramsoc.2019.04.027.
- Lei Han; Cui Li; Chang-Wei Lin; Jianlei Liu; Jia-Qi Wu; Hua Gui; Qian Zhang; Zhi-Wei Luo; Tao-Yong Liu; Anxian Lu; et al. A novel Nd3+ ‐doped MgO‐Al2O3‐SiO2‐based transparent glass‐ceramics: Toward excellent fluorescence properties. Journal of the American Ceramic Society 2019, 102, 4213-4225, 10.1111/jace.16306.
- Zanotto, Edgar Dutra; A bright future for glass-ceramics. AMERICAN CERAMIC SOCIETY BULLETIN 2011, 6, 609-612, 10.2217/fmb.11.55.
- George H. Beall; Milestones in Glass-Ceramics: A Personal Perspective. International Journal of Applied Glass Science 2014, 5, 93-103, 10.1111/ijag.12063.
- Larry L. Hench; Julia Polak; Third-Generation Biomedical Materials. Science 2002, 295, 1014-1017, 10.1126/science.1067404.
- E. El-Meliegy, R. Van Noort. Glasses and glass ceramics for medical applications; Springer: London, 2012; pp. 14.
- João S. Fernandes; Piergiorgio Gentile; Ricardo A. Pires; Rui L. Reis; Paul Hatton; Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomaterialia 2017, 59, 2-11, 10.1016/j.actbio.2017.06.046.
- Yasushi Inda; Takashi Katoh; Mamoru Baba; Development of all-solid lithium-ion battery using Li-ion conducting glass-ceramics. Journal of Power Sources 2007, 174, 741-744, 10.1016/j.jpowsour.2007.06.234.
- Zhijun Wu; Zhengkun Xie; Akihiro Yoshida; Zhongde Wang; Xiaogang Hao; Abuliti Abudula; Guoqing Guan; Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renewable and Sustainable Energy Reviews 2019, 109, 367-385, 10.1016/j.rser.2019.04.035.
- P.F. James; Glass ceramics: new compositions and uses. Journal of Non-Crystalline Solids 1995, 181, 1-15, 10.1016/0022-3093(94)00515-x.
- P.F. James; Glass ceramics: new compositions and uses. Journal of Non-Crystalline Solids 1995, 181, 1-15, 10.1016/0022-3093(94)00515-x.
