Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer is a global burden, and as per the latest GLOBOCAN 2020, over 19.3 and 10 million new cases and deaths occurred in 2020, respectively; female breast cancer has surpassed lung cancer and is now the most commonly diagnosed cancer (11.7%), followed by lung cancer (11.4%), colorectal cancer (10%), and prostate cancer (7.3%). Cancer chemoresistance is a growing concern in medical oncology.
- cancer
- chemotherapy
- multidrug resistance
- nanotechnology
1. Introduction
Cancer is a global burden, and as per the latest GLOBOCAN 2020, over 19.3 and 10 million new cases and deaths occurred in 2020, respectively; female breast cancer has surpassed lung cancer and is now the most commonly diagnosed cancer (11.7%), followed by lung cancer (11.4%), colorectal cancer (10%), and prostate cancer (7.3%) [1]. In mortality, lung cancer remains at the top [1]. As per World Health Organization (WHO) statistics 2019, in 112 out of 183 countries in the world, people die of cancer before attaining the age of 70 years [2]. Despite the world having advanced in science and technology, chemotherapy remains a promising option to treat cancer [3]. Conventional chemotherapy has greatly improved the decline in the mortality rate of several dreadful cancers, but its major problem is the killing of cancerous and noncancerous cells causing serious off-target effects such as hair loss, bone marrow depression, and other toxic effects [4]. Therefore, a significant percentage of cancer-related research over the past few decades has focused on creating medications that more precisely target tumor cells rather than normal cells [5]. Precision therapy has greatly advanced thanks to the development of targeted therapy, but there are still numerous unavoidable side effects, and drug resistance has long been an issue [6].
Over 90% of failures in chemotherapy are due to the development of resistance to the already available drugs; this resistance resembles infectious disease treatment resistance and is the most challenging aspect of treating and preventing cancers [7]. This has emerged as a major obstacle and allows cancer to proliferate in presence of a chemotherapeutic agent [8]. Significant resistance develops generally to repeated treatment with one kind of anticancer agent and then develops further towards similar or completely different drugs having a similar mechanism of action. This mechanism, known as multidrug resistance (MDR), can be intrinsic or acquired [9].
To overcome this problem, in recent years, nanotechnology-based drug dosage forms have been explored, which have shown great promise [10]. Most of these nanomedicines are heading toward clinical trials [11]. Nanotechnology has been used in medicine more and more over the past few decades, including applications for safer and more efficient tumor targeting, detection, and treatment [12][13][14][15][16][17][18][19][20]. Drug delivery methods based on nanoparticles (NPs) have demonstrated a number of benefits in the treatment of cancer, including good pharmacokinetics, accurate targeting of tumor cells, a decrease in adverse effects, and reduced drug resistance [21][22][23][24][25]. Nevertheless, nanomedicine-based formulations have some demerits, such as difficulty in physical handling due to smaller size, particle aggregation, limited drug loading, and burst release [19][20].
2. Cancer Chemotherapy Resistance and Mechanism
Cancer chemoresistance is a growing concern in medical oncology. Some cancers, including Hodgkin’s lymphomas, acute promyelocytic leukemia, and chronic myeloid leukemia, have been successfully understood and treated despite their complex pathophysiology [26]. The development of anticancer agents against these complex cancers has been achieved by understanding the deep mechanisms, and various drugs have been developed [7]. These mainly include the stimulation of immune response using interferon-alpha (IF-α) and inhibition of oncogenes or oncoproteins [27][28][29][30][31][32][33][34][35]. Many of them are still in practice; however, resistance has developed to the majority of them, which has ultimately affected patient survival [36]. There are various reported resistance mechanisms associated with cancer chemotherapy such as drug efflux, detoxification, stem cells, epithelial-to-mesenchymal transition, inactivation of the drugs before reaching the target, multidrug resistance, inhibiting cell death (apoptosis suppression), augmenting gene amplification and DNA repair of oncogenes, and alteration in the metabolism of drugs. Figure 1 shows the illustration of different possible mechanisms of chemotherapy resistance [37]. Drug resistance in cancer is believed to be due to intrinsic and acquired resistance; however, most cancers in clinical settings have become resistant owing to combinations of these factors [38].
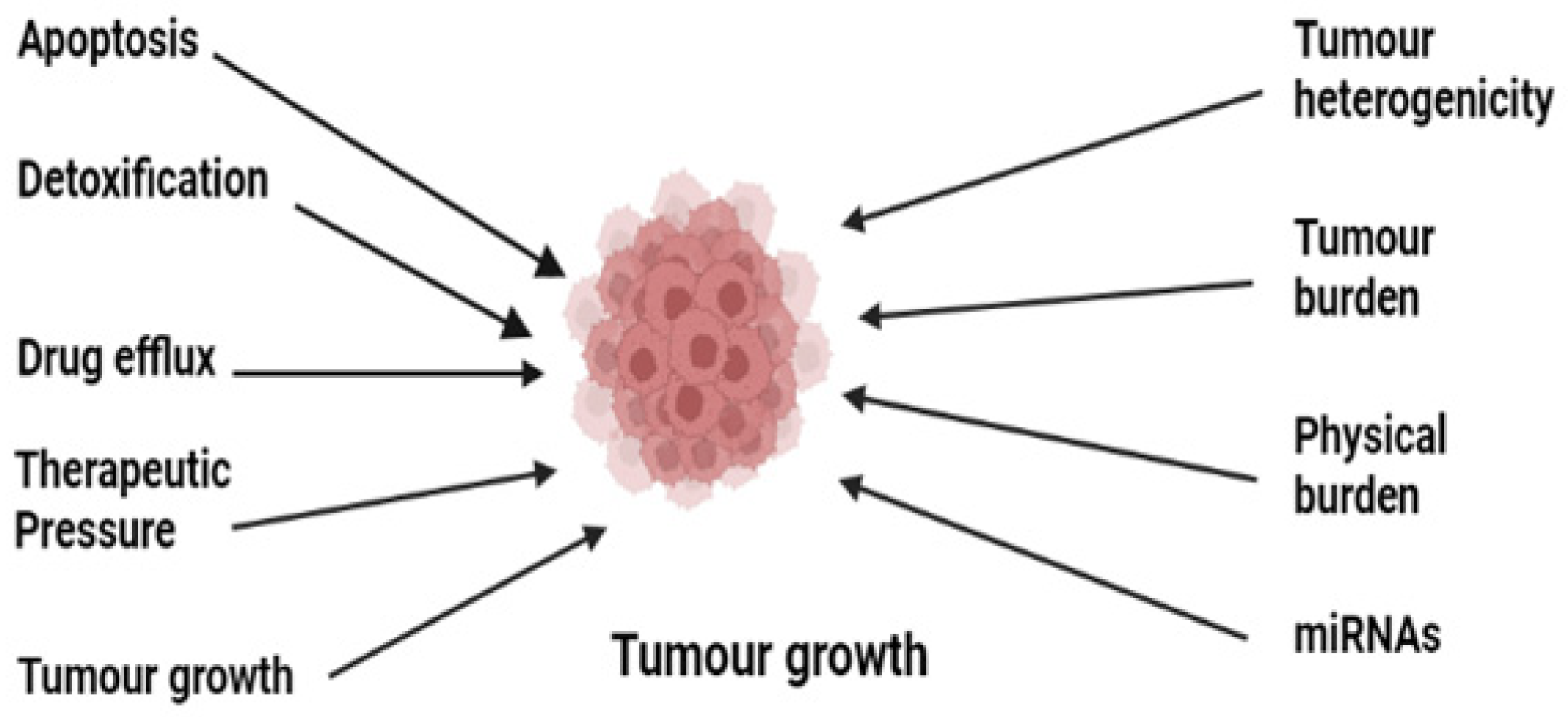
Figure 1. Illustration of the various possible underlying mechanisms in the development of drug resistance in cancer.
2.1. Role of Drug Efflux Pumps in Cancer Drug Resistance
The human genome encodes 48 members of drug efflux proteins called ATP-binding cassettes which are further classified into seven subgroups (ABCA, ABCB ABCC, ABCD, ABCE, ABCF, and ABCG) [39]. These proteins have a significant role in the development of drug resistance [40]. These proteins expel the drug out of the cell, thereby reducing the therapeutic concentration of the drug inside the cell [41]. Enough evidence suggests the overexpression of these proteins, especially multiple drug resistance protein 1 (MDR 1) known as P-glycoprotein, multiple drug resistance-associated protein (MDRA), and breast cancer resistant protein (BCRP), on cellular surfaces [42]. Normally these transporters help in pumping out toxins and foreign substances [43]. These transporters in general and P-pg in particular transport a range of substances including anticancer agents out of the cell, causing depletion of therapeutic concentration. Overexpression of P-pg in patients causes efflux of paclitaxel and doxorubicin and leads to resistance to these drugs [44]. This is evidenced by a study conducted on a genetically engineered mouse model (GEMM), where the tumor recurred due to upregulation of ABCB1a and b and was found to be cross-resistant to docetaxel also [39][45]. Another study shows the non-responsiveness of the tumor to olaparib, a PARP inhibitor, due to the overexpression of ABC1 a/b [46][47], thus confirming the overexpression of these efflux proteins in drug-resistant cancers.
2.2. Suppression of Apoptosis
Although apoptosis and autophagy are altogether different, they ultimately contribute to cell death [48]. Two different mechanisms in apoptosis contribute to cell death: (a) intrinsic, which involves the mitochondrial-mediated bcl2 proteins, Akt, and caspase-9, and (b) extrinsic, which involves death receptors on the cellular surface [49]. Ample evidence supports the initiation of human cancer from cancer stem cells (CSCs), and it is believed that these apoptotic pathways become dysregulated and lead to cancer chemotherapy resistance and tumor recurrence [50]. High levels of antiapoptotic proteins which are considered the hallmarks of cancer have been seen in drug-resistant cancers [51]. The antiapoptotic protein family which includes Bcl-2, Mcl-1, and Bcl-xL has been seen at raised levels compared to proapoptotic proteins Bax, Puma, Noxa, Bak, Bil, and Bid, causing an imbalance between the pro- and antiapoptotic proteins which ultimately leads to cancer drug resistance [52]. The formation of the mitochondrial apoptosis-induced channel (MAC), which is formed by binding of tBid with Bax and Bak through activated caspase-8, is hindered by the downregulation of proapoptotic and upregulation of antiapoptotic proteins, which leads to the formation of resistant cancers by inhibiting the release of cytochrome C, a key protein for electron transfer in mitochondria [53]. This overexpression of antiapoptotic proteins is responsible for drug resistance in multiple cancers [54]. Additionally, overexpression of Nf-kB, P53, and PI3/AKT cell death-related receptors is also involved in chemoresistance [55]. In addition, apoptosis evasion through aberrant autophagy is another factor in the development of multiple drug resistance [56].
2.3. Drug Inactivation
Before a drug reaches the gastrointestinal tract or systemic circulation, some drugs that are in prodrug form interact with certain proteins which partially degrade, modify, and form complexes with other endogenous substances, leading to the activation of a drug [57]. Certain cancers have developed resistance due to decreased activation of prodrugs to active drugs [58]; the most prominent example is the mutation and downregulation of phosphorylation events in the conversion of AraC into AraC-triphosphate which is used in the treatment of acute myelogenous leukemia [8][59]. Several drugs metabolizing enzymes such as uridine diphosphate-glucuronosyltransferase, the glutathione-S-transferase family, and cytochrome P450 are muted one way or another and ultimately lead to resistance to already available drugs [60]. The overactivity of cytochrome p450 has been reported to lead to its resistance in breast cancer [61]. Detoxification of drugs by overproduction of glutathione has led to the development of resistance to many platinum compounds and alkylating agents such as cisplatin and doxorubicin [62]. Thus, mutations in phase I and phase II reactions either reduce the activity of drugs by increasing their detoxification or lead to the development of drug resistance by inactivating certain drugs.
2.4. Role of miRNAs in Cancer Drug Resistance
miRNAs are processed from RNA hairpin structures, which regulate genes in cancer, especially in resistant ones [63]. They are involved in apoptosis, cellular proliferation, stress tolerance, the cell cycle, and immune response [64]. Around 30% of human genes are regulated by miRNAs and have a role in tumor development [63]. Some act as protumor genes, some act as suppressor genes, and some act as both [65]. Studies conducted by various researchers provide evidence of miRNAs being involved in cancer drug resistance by either enhancing tumor cancer genes or having involvement in genes that are related to apoptosis, cellular proliferation, and the cell cycle [66]. Due to their tissue specificity, one kind of microRNA could be targeted by multiple microRNAs; hence the same miRNA can either promote or inhibit resistance to chemotherapy [67][68]. In breast cancer, upregulation of miRNA-21 downregulates phosphatase tensin homolog (PTEN) and thereby decreases the susceptibility of doxorubicin to cancer cells, while overexpression of PTEN inhibits miRNA-21 and reduces the resistance of breast cancer cells to doxorubicin [69]. Table 1 reports some of the miRNAs that regulate cancer chemotherapeutic drug resistance [70][71][72][73][74][75][76][77][78][79][80].
Table 1. List of some miRNAs that regulate cancer chemoresistance.
| miRNA | Target | Cancer Type | Drug Target | Reference |
|---|---|---|---|---|
| miR-7 | MDR1 | SCLC | Anthracyclines | [70] |
| miR-21 | PTEN | Breast | Trastuzumab | [71] |
| miR-20a | MAPK1 | Colorectal | 5-Fluorouracil | [72] |
| miR-103/107 | P-gp | Gastric | Doxorubicin | [73] |
| miR-196a | MDR1/MRP1 | NSCLC | Cisplatin | [74] |
| miR-17-5p | PHIPP2 | MCL | Topotecan | [75] |
| microRNA-34a | SIRT1, Bcl-2 | Prostate | Paclitaxel | [76] |
| miR-96 | XIAP | Colorectal | 5-Fluorouracil | [77] |
| miR-499a | UBE2V2 | Cervical | 5-Fluorouracil | [78] |
| miR-RNA-449 | NOTCH1 | Ovarian | Doxorubicin | [79] |
| miR-320c | SMARCC1 | Pancreatic | Gemcitabine | [80] |
Abbreviations: MDR1: multidrug resistance mutation 1; PTEN: phosphatase tensin homolog; MAPK1: mitogen-activated protein kinase 1; P-gp: P-glycoprotein; MRP1: multidrug resistance-associated protein 1; PHIPP2: phage phi-PP2; SIRT1: sirtuin 1; XIAP: X-linked inhibitor of apoptosis protein; Bcl-2: B-cell lymphoma-2; UBE2V2: ubiquitin conjugated enzyme E2V2; NOTCH1: human gene; SMARCC1: protein; SCLC: small cell lung carcinoma; NSCLC: non-small-cell lung carcinoma; MCL: mantle cell lymphoma.
2.5. Tumor Microenvironment (TME)
The TME leads to the development of resistance by providing an environment rich in the stroma, immune cells, and vasculature which helps in the development of resistance by several mechanisms such as hampering drug absorption, restricting immune clearing of cancer cells, and stimulating factors for cancer cell proliferation [81]. Lactic acid produced by intermediate glycolytic intermediates results in a change in pH in cancer cells; this change in pH gradient results in neutralization and protonation of certain anticancer agents such as doxorubicin, thereby preventing them from entering the target site [82][83]. Hypoxia is a key factor in cancer drug resistance; its transcription factor hypoxia-inducible factor (HIF) is expressed in many cancers [84], which is supported by numerous studies involving HIF inhibitors as chemosensitizing agents in cancer. The TME also helps tumors to become resistant by providing an environment for metabolic reprogramming, DNA repair, and the immune microenvironment [85].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196608
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030.
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300.
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7.
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73.
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, E22.
- Goodman, L.S.; Wintrobe, M.M. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946, 132, 126–132.
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792.
- Ozben, T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006, 580, 2903–2909.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790.
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973.
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhnacement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C 2017, 76, 319–329.
- Alshahrani, S.M.; Alshetaili, A.S.; Alalaiwe, A.; Alsulays, B.B.; Anwer, M.K.; Al-Shdefat, R.; Imam, F.; Shakeel, F. Anticancer Efficacy of Self-Nanoemulsifying Drug Delivery System of Sunitinib Malate. AAPS PharmSciTech 2018, 19, 123–133.
- Shazly, G.A.; AlShehri, S.; Ibrahim, M.A.; Tawfeek, H.M.; Razik, J.A.; Hassan, Y.A.; Shakeel, F. Development of Domperidone Solid Lipid Nanoparticles: In Vitro and In Vivo Characterization. AAPS PharmSciTech 2018, 19, 1712–1719.
- Hussain, A.; Shakeel, F.; Singh, S.K.; Alsarra, I.A.; Faruk, A.; Alanazi, F.K.; Christoper, G.P. Solidified SNEDDS for the oral delivery of rifampicin: Evaluation, proof of concept, in vivo kinetics, and in silico GastroPlusTM simulation. Int. J. Pharm. 2019, 566, 203–217.
- Kazi, M.; Alhajri, A.; AlShehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749.
- Abushal, A.S.; Aleanizy, F.S.; Alqahtani, F.Y.; Shakeel, F.; Iqbal, M.; Haq, N.; Alsarra, I.A. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of Apremilast: In Vitro Evaluation and Pharmacokinetics Studies. Molecules 2022, 27, 3085.
- Soliman, N.M.; Shakeel, F.; Haq, N.; Alanazi, F.K.; Alshehri, S.; Bayomi, M.; Alenazi, A.S.M.; Alsarra, I.A. Development and Optimization of Ciprofloxacin HCl-Loaded Chitosan Nanoparticles Using Box–Behnken Experimental Design. Molecules 2022, 27, 4468.
- Shoaib, A.; Azmi, L.; Pal, S.; Alqahtani, S.S.; Rahamathulla, M.; Hani, U.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Integrating nanotechnology with naturally occurring phytochemicals in neuropathy induced by diabetes. J. Mol. Liq. 2022, 350, 118189.
- Badran, M.M.; Mady, M.M.; Ghannam, M.M.; Shakeel, F. Preparation and characterization of polymeric nanoparticles surfacemodified with chitosan for target treatment of colorectal cancer. Int. J. Biol. Macromol. 2017, 95, 643–649.
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305.
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.; Alqahtani, S.; Kazi, M.; Shakeel, F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics 2021, 13, 368.
- Alshammari, R.A.; Aleanizy, F.S.; Aldarwesh, A.; Alqahtani, F.Y.; Mahdi, W.A.; Alquadeib, B.; Alqahtani, Q.H.; Haq, N.; Shakeel, F.; Abdelhady, H.G.; et al. Retinal Delivery of the Protein Kinase C-β Inhibitor Ruboxistaurin Using Non-Invasive Nanoparticles of Polyamidoamine Dendrimers. Pharmaceutics 2022, 14, 1444.
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A review on nanotechnology: Properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 2022, 348, 118008.
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA A Cancer J. Clin. 2012, 62, 220–241.
- Raderer, M.; Scheithauer, W. Treatment of advanced colorectal cancer with 5-fluorouracil and interferon-α: An overview of clinical trials. Eur. J. Cancer 1995, 31, 1002–1008.
- Guilhot, F.; Chastang, C.; Michallet, M.; Guerci, A.; Harousseau, J.-L.; Maloisel, F.; Bouabdallah, R.; Guyotat, D.; Cheron, N.; Nicolini, F.; et al. Interferon Alfa-2b Combined with Cytarabine versus Interferon Alone in Chronic Myelogenous Leukemia. New Engl. J. Med. 1997, 337, 223–229.
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037.
- Tallman, M.S.; Nabhan, C.; Feusner, J.H.; Rowe, J.M. Acute promyelocytic leukemia: Evolving therapeutic strategies. Blood 2002, 99, 759–767.
- O’Brien, S.G.; Guilhot, F.; Larson, R.A.; Gathmann, I.; Baccarani, M.; Cervantes, F.; Cornelissen, J.J.; Fischer, T.; Hochhaus, A.; Hughes, T.; et al. Imatinib Compared with Interferon and Low-Dose Cytarabine for Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2003, 348, 994–1004.
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297.
- Ferrantini, M.; Capone, I.; Belardelli, F. Interferon-α and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie 2007, 89, 884–893.
- Chin, L.; Gray, J.W. Translating insights from the cancer genome into clinical practice. Nature 2008, 452, 553–563.
- Sellers, W.R. A Blueprint for Advancing Genetics-Based Cancer Therapy. Cell 2011, 147, 26–31.
- Wilson, T.; Johnston, P.; Longley, D. Anti-Apoptotic Mechanisms of Drug Resistance in Cancer. Curr. Cancer Drug Targets 2009, 9, 307–319.
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292.
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228.
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234.
- Fernández, L.; Hancock, R.E.W. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin. Microbiol. Rev. 2012, 25, 661–681.
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58.
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30.
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2019, 2, 141–160.
- Kersten, K.; Visser, K.E.; Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153.
- Choi, Y.E.; Meghani, K.; Brault, M.-E.; Leclerc, L.; He, Y.; Day, T.A.; Elias, K.M.; Drapkin, R.; Weinstock, D.M.; Dao, F.; et al. Platinum and PARP Inhibitor Resistance Due to Overexpression of MicroRNA-622 in BRCA1-Mutant Ovarian Cancer. Cell Rep. 2016, 14, 429–439.
- Weil, M.K.; Chen, A.P. PARP Inhibitor Treatment in Ovarian and Breast Cancer. Curr. Probl. Cancer 2011, 35, 7–50.
- Chen, L.; Zeng, Y.; Zhou, S.-F. Role of Apoptosis in Cancer Resistance to Chemotherapy. Program. Cell Death. InTech. 2018.
- Fulda, S.; Debatin, K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811.
- Safa, A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016, 21, 203–219.
- Llambi, F.; Green, D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011, 21, 12–20.
- Liu, B.; Yuan, B.; Zhang, L.; Mu, W.; Wang, C. ROS/p38/p53/Puma signaling pathway is involved in emodin-induced apoptosis of human colorectal cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 15413–15422.
- Meng, X.; Carlson, N.R.; Dong, J.; Zhang, Y. Oncogenic c-Myc-induced lymphomagenesis is inhibited non-redundantly by the p19Arf-Mdm2-p53 and RP-Mdm2-p53 pathways. Oncogene 2015, 34, 5709–5717.
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964.
- Yang, H.-J.; Wang, M.; Wang, L.; Cheng, B.-F.; Lin, X.-Y.; Feng, Z.-W. NF-κB regulates caspase-4 expression and sensitizes neuroblastoma cells to Fas-induced apoptosis. PLoS ONE 2015, 10, E0117953.
- Rubinstein, A.D.; Kimchi, A. Life in the balance—A mechanistic view of the crosstalk between autophagy and apoptosis. J. Cell Sci. 2012, 125, 5259–5268.
- Zubay, G.; Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001, 344, 1038–1042.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues: Mechanisms of drug resistance and reversal strategies. Leukemia 2001, 15, 875–890.
- Longo-Sorbello, G.S.; Bertino, J.R. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica 2001, 86, 121–127.
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955.
- Jansen, B.A.; Brouwer, J.; Reedijk, J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J. Inorg. Biochem. 2002, 89, 197–202.
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587.
- Schickel, R.; Boyerinas, B.; Park, S.-M.; Peter, M.E. MicroRNAs: Key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 2008, 27, 5959–5974.
- Jones, M.F.; Lal, A. MicroRNAs, wild-type and mutant p53: More questions than answers. RNA Biol. 2012, 9, 781–791.
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32.
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63.
- Wu, S.; Huang, S.; Ding, J.; Zhao, Y.; Liang, L.; Liu, T.; Zhan, R.; He, X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene 2010, 29, 2302–2308.
- Wang, Z.-X.; Lu, B.-B.; Wang, H.; Cheng, Z.-X.; Yin, Y.-M. MicroRNA-21 Modulates Chemosensitivity of Breast Cancer Cells to Doxorubicin by Targeting PTEN. Arch. Med. Res. 2011, 42, 281–290.
- Liu, H.; Wu, X.; Huang, J.; Peng, J.; Guo, L. miR-7 modulates chemoresistance of small cell lung cancer by repressing MRP1/ABCC1. Int. J. Exp. Pathol. 2015, 96, 240–247.
- De Mattos-Arruda, L.; Bottai, G.; Nuciforo, P.G.; Di Tommaso, L.; Giovannetti, E.; Peg, V.; Losurdo, A.; Pérez-Garcia, J.; Masci, G.; Corsi, F.; et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget 2015, 6, 37269–37280.
- Si, W.; Shen, J.; Du, C.; Chen, D.; Gu, X.; Li, C.; Yao, M.; Pan, J.; Cheng, J.; Jiang, D.; et al. A miR-20a/MAPK1/c-Myc regulatory feedback loop regulates breast carcinogenesis and chemoresistance. Cell Death Differen. 2018, 25, 406–420.
- Zhang, Y.; Qu, X.; Li, C.; Fan, Y.; Che, X.; Wang, X.; Cai, Y.; Hu, X.; Liu, Y. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumor Biol. 2015, 36, 2277–2285.
- Li, J.-H.; Luo, N.; Zhong, M.-Z.; Xiao, Z.-Q.; Wang, J.-X.; Yao, X.-Y.; Peng, Y.; Cao, J. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumor Biol. 2016, 37, 2387–2394.
- Rao, E.; Jiang, C.; Ji, M.; Huang, X.; Iqbal, J.; Lenz, G.; Wright, G.; Staudt, L.M.; Zhao, Y.; McKeithan, T.; et al. The miRNA-17∼92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia 2012, 26, 1064–1072.
- Liu, X.; Luo, X.; Wu, Y.; Xia, D.; Chen, W.; Fang, Z.; Deng, J.; Hao, Y.; Yang, X.; Zhang, T.; et al. MicroRNA-34a attenuates paclitaxel resistance in prostate cancer cells via direct suppression of JAG1/notch1 axis. Cell. Physiol. Biochem. 2018, 50, 261–276.
- Kim, S.-A.; Kim, I.; Yoon, S.K.; Lee, E.K.; Kuh, H.-J. Indirect modulation of sensitivity to 5-fluorouracil by microRNA-96 in human colorectal cancer cells. Arch. Pharmacal Res. 2014, 38, 239–248.
- Chen, Y.; Song, Y.; Mi, Y.; Jin, H.; Cao, J.; Li, H.; Han, L.; Huang, T.; Zhang, X.; Ren, S.; et al. microRNA-499a promotes the progression and chemoresistance of cervical cancer cells by targeting SOX6. Apoptosis 2020, 25, 205–216.
- Tormo, E.; Ballester, S.; Adam-Artigues, A.; Burgués, O.; Alonso, E.; Bermejo, B.; Menéndez, S.; Zazo, S.; Madoz-Gúrpide, J.; Rovira, A.; et al. The miRNA-449 family mediates doxorubicin resistance in triple-negative breast cancer by regulating cell cycle factors. Sci. Rep. 2019, 9, E5316.
- Iwagami, Y.; Eguchi, H.; Nagano, H.; Akita, H.; Hama, N.; Wada, H.; Kawamoto, K.; Kobayashi, S.; Tomokuni, A.; Tomimaru, Y.; et al. miR-320c regulates gemcitabine-resistance in pancreatic cancer via SMARCC1. Br. J. Cancer 2013, 109, 502–511.
- Li, Z.-W.; Dalton, W.S. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006, 20, 333–342.
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218.
- Choi, S.Y.C.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013, 230, 350–355.
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10.
- Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins As Central Mediators of Metabolism in the Tumor Cells and Their Microenvironment. Front. Immunol. 2018, 9, 40.
This entry is offline, you can click here to edit this entry!
