Heterogeneous catalysis (HC) has become a valuable means for designing sustainable production protocols to obtain superior intermediates, synthons, and potent bioactive heterocycles. The heterogeneous catalysts proved economical and eco-friendly due to the effectiveness of non-hazardous metals for constructing nanomaterials for atom-economical organic reactions. Heterogeneous catalysts significantly affected scientific and industrial innovations due to their tuneable textural properties, flexible acid-base characteristics, higher thermal stability, and improved reusability, with their excellent performance over their enormous counterparts.
- green synthesis
- single-step synthesis
- pyrans
1. Introduction
2. Synthesis of Pyran Derivatives
2.1. Iron Base Catalysts
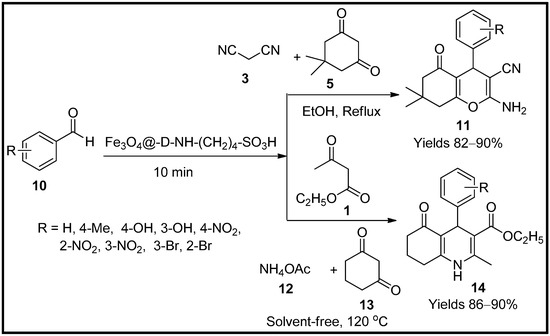
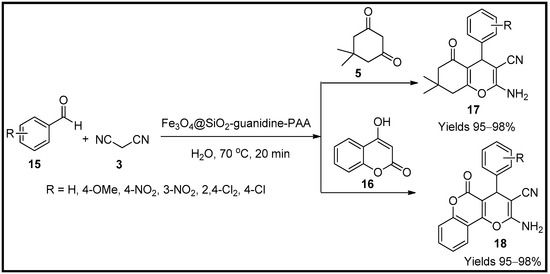
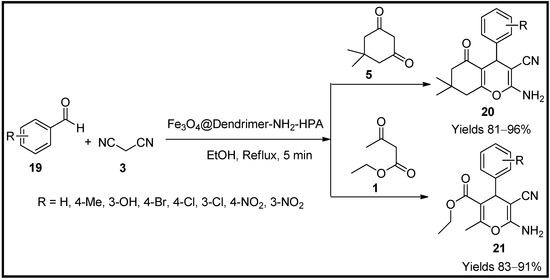
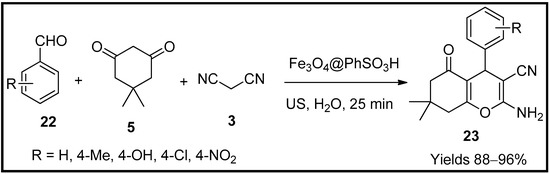
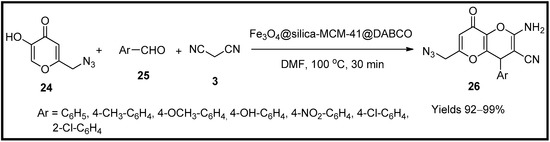
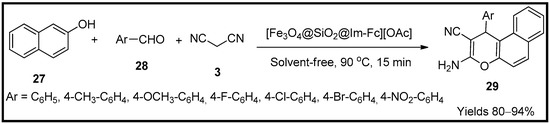
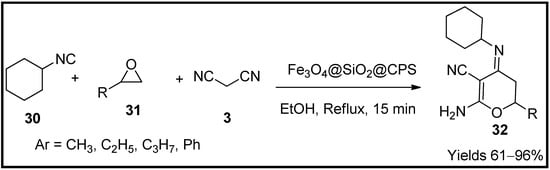
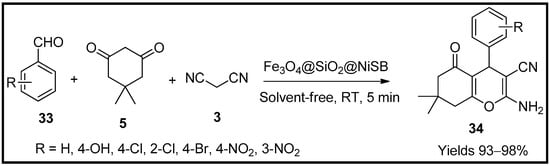
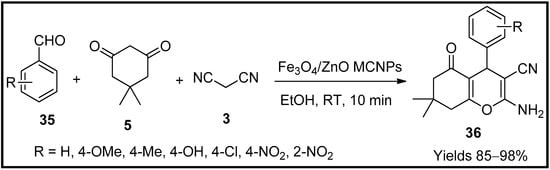
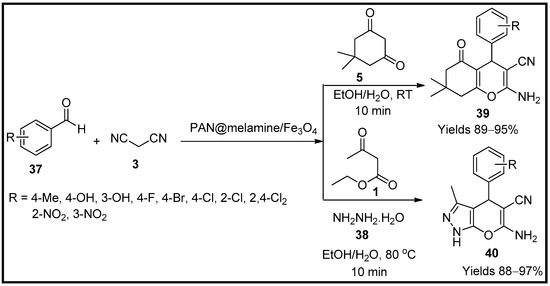
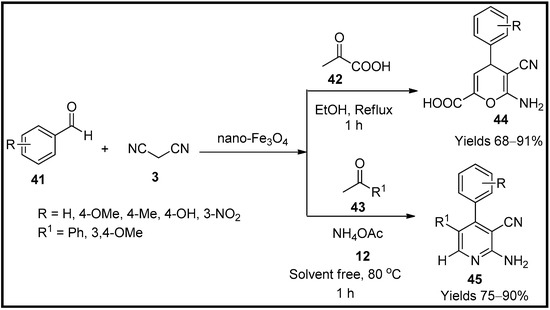
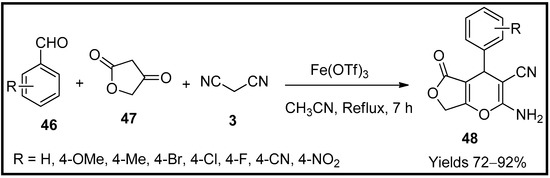
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196347
References
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359.
- Vedrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341.
- Kerru, N.; Gummidi, L.; Maddila, S.; Jonnalagadda, S.B. A review of recent advances in the green synthesis of azole- and pyran based fused heterocycles using MCRs and sustainable catalysts. Curr. Org. Chem. 2021, 25, 4–39.
- Kerru, N.; Bhaskaruni, S.V.H.S.; Gummidi, L.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Syn. Commun. 2019, 49, 2437–2459.
- Bhaskaruni, S.V.H.S.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on multicomponent green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arab. J. Chem. 2020, 13, 1142–1178.
- Hutchings, G.J. Heterogeneous catalysts-discovery and design. J. Mater. Chem. 2009, 19, 1222–1235.
- Climent, M.J.; Corma, A.; Iborra, S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012, 2, 16–58.
- Harikrishna, S.; Gangu, K.K.; Robert, A.R.; Ganja, H.; Kerru, N.; Jonnalagadda, S.B. An eco-friendly and reusable catalyst RuO2/MWCNT in the green synthesis of sulfonyl-quinolines. Process Saf. Environ. Prot. 2022, 159, 911–917.
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. The pioneering role of metal–organic framework-5 in ever-growing contemporary applications. RSC Adv. 2022, 12, 14282–14298.
- Ganta, R.K.; Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Advances in pyranopyrazole scaffolds' syntheses using sustainable catalysts-a review. Molecules 2021, 26, 3270.
- Kerru, N.; Gummidi, L.; Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. Synthesis of novel furocoumarin derivatives through multicomponent cycloaddition reaction using ZnO/FAp as a sustainable catalyst. ChemistrySelect 2020, 5, 4104–4110.
- Kerru, N.; Gummidi, L.; Maddila, S.; Jonnalagadda, S.B. Efficient synthesis of novel functionalised dihydro-pyrazolo-pyridines via the three-component reaction using MgO/HAp as a sustainable catalyst. Inorg. Chem. Commun. 2021, 123, 108321.
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. Green synthesis and characterisation of novel 1,2,4,5-tetrasubstituted imidazole derivatives with eco-friendly red brick clay as efficacious catalyst. Mol. Divers. 2020, 24, 889–901.
- Kerru, N.; Gummidi, L.; Maddila, S.; Jonnalagadda, S.B. Gadolinium oxide loaded zirconia and multicomponent synthesis of novel dihydro-pyrazolopyridines under green conditions. Sustain. Chem. Pharm. 2020, 18, 100316.
- Kerru, N.; Gummidi, L.; Maddila, S.N.; Bhaskaruni, S.V.H.S.; Maddila, S.; Jonnalagadda, S.B. Green synthesis and characterisation of novel -thiadiazolo/benzothiazolopyrimidines via multicomponent reaction using vanadium oxide loaded on fluorapatite as a robust and sustainable catalyst. RSC Adv. 2020, 10, 19803–19810.
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. A facile and catalyst-free microwave promoted multicomponent reaction for the synthesis of functionalised 1,4-dihydropyridines with superb selectivity and yields. Front. Chem. 2021, 9, 638832.
- Suryanarayana, K.; Robert, A.R.; Kerru, N.; Pooventhiran, T.; Thomas, R.; Maddila, S.; Jonnalagadda, S.B. Design, synthesis, anticancer activity and molecular docking analysis of novel dinitrophenylpyrazole bearing 1,2,3-triazoles. J. Mol. Struct. 2021, 1243, 130865.
- Rao, B.S.; Reddy, K.V.N.S.; Kerru, N.; Maddila, S. An efficient synthesis of drug-like small molecules library based on 2-(substituted benzylthio)-4,6-dichloropyrimidin-5-amines. Chem. Data Coll. 2021, 33, 100704.
- Muralidhar, P.; Kumar, B.S.; Kerru, N.; Maddila, S. A novel method for the synthesis of 3-aminoindoles using iodine and Cs2CO3 as catalyst. Chem. Data Coll. 2021, 33, 100731.
- Maddila, S.; Devi, L.; Muralidhar, P.; Nagaraju, K.; Jonnalagadda, S.B. A facile and environmental-friendly protocol for the synthesis of methyleneisoxazole-5(4H)-ones catalysed by CeO2/TiO2 under ultrasonic irradiation. Inorg. Chem. Commun. 2022, 143, 109741.
- Madhavi, C.; Robert, A.R.; Gangu, K.K.; Kerru, N.; Maddila, S. An efficient and sustainable synthesis of morpholino-1,4-dihydropyridine-2,3-dicarboxylates using recyclable SeO2/HAp catalyst. Inorg. Chem. Commun. 2022, 143, 109750.
- Kerru, N.; Gummidi, L.; Maddila, S.N.; Bhaskaruni, S.V.H.S.; Jonnalagadda, S.B. Ultrasound-assisted synthesis and anti-bacterial activity of novel 1,3,4-thiadiazole-1H-pyrazol-4-yl-thiazolidin-4-one derivatives. Monatsh. Chem. 2020, 151, 981–990.
- Kerru, N.; Gummidi, L.; Maddila, S.N.; Bhaskaruni, S.V.H.S.; Jonnalagadda, S.B. Bi2O3/FAp, a sustainable catalyst for synthesis of dihydro-triazolopyrimidine derivatives through green strategy. Appl. Organometal. Chem. 2020, 34, e5590.
- Kerru, N.; Gummidi, L.; Maddila, S.; Jonnalagadda, S.B. Polyethylene glycol (PEG-400) mediated one-pot green synthesis of 4,7-dihydro-2H-pyrazolopyridines under catalyst-free conditions. ChemistrySelect 2020, 5, 12407–12410.
- Slobbe, P.; Ruijter, E.; Orru, R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun. 2012, 3, 1189–1218.
- Maddila, S.; Kerru, N.; Jonnalagadda, S.B. Synthesis and antimicrobial evaluation of novel pyrano-pyrimidine bearing 1,2,3-triazoles. Chem. Data Coll. 2020, 6, 100486.
- Madhavi, C.; Ganja, H.; Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Synthesis of a sustainable heterogeneous catalyst, titanium dioxide-loaded hydroxyapatite for functionalized chromen-dihydropyridines under green conditions. Appl. Organomet. Chem. 2021, 34, e6442.
- Devi, L.; Kerru, N.; Maddila, S.; Jonnalagadda, S.B. A green, efficient protocol for the catalyst-free synthesis of tetrahydro-1H-pyrazolo--quinolin-5(4H)-ones supported by ultrasonicirradiation. Chem. Data Coll. 2020, 30, 100566.
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multi-component reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975.
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. One-pot green synthesis of novel 5,10-dihydro-1H-pyrazolophthalazine derivatives with eco-friendly biodegradable eggshell powder as efficacious catalyst. Res. Chem. Intermed. 2020, 46, 3067–3083.
- Kerru, N.; Gummidi, L.; Maddila, S.N.; Gangu, K.K.; Jonnalagadda, S.B. Four-component rapid protocol with nickel oxide loaded on fluorapatite as a sustainable catalyst for the synthesis of novel imidazole analogs. Inorg. Chem. Commun. 2020, 116, 107935.
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128.
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278.
- Ferrazzano, L.; Corbisiero, D.; Martelli, G.; Tolomelli, A.; Viola, A.; Ricci, A.; Cabri, W. Green solvent mixtures for solid-phase peptide synthesis: A dimethylformamide-free highly efficient synthesis of pharmaceutical-grade peptides. ACS Sustain. Chem. Eng. 2019, 7, 12867–12877.
- Maddila, S.; Nagaraju, K.; Chinnam, S.; Jonnalagadda, S.B. Microwave-assisted multicomponent reaction: A green and catalyst-free method for the synthesis of poly-functionalised 1,4-dihydropyridines. ChemistrySelect 2019, 4, 9451–9454.
- Kerru, N.; Bhaskaruni, S.V.H.S.; Gummidi, L.; Maddila, S.N.; Singh, P.; Jonnalagadda, S.B. Efficient synthesis of novel pyrazole linked 1,2,4-triazolidine-3-thiones using bismuth on zirconium oxide as a recyclable catalyst in aqueous medium. Mol. Divers. 2019, 24, 345–354.
- Kerru, N.; Bhaskaruni, S.V.H.S.; Gummidi, L.; Maddila, S.N.; Rana, S.; Singh, P.; Jonnalagadda, S.B. Synthesis of novel pyrazolebased triazolidin-3-one derivatives by using ZnO/ZrO2 as a reusable catalyst under green conditions. Appl. Organometal. Chem. 2019, 33, e4722.
- Paplal, B.; Nagaraju, S.; Veerabhadraiah, P.; Sujatha, K.; Kanvah, S.; Kumar, B.V.; Kashinath, D. Recyclable Bi2WO6-nanoparticle mediated one-pot multicomponent reactions in aqueous medium at room temperature. RSC Adv. 2014, 4, 54168–54174.
- Nagaraju, S.; Paplal, B.; Sathish, K.; Giri, S.; Kashinath, D. Synthesis of functionalised chromene and spirochromenes using l-proline-melamine as highly efficient and recyclable homogeneous catalyst at room temperature. Tetrahedron Lett. 2017, 58, 4200–4204.
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909.
- Rao, D.J.; Kerru, N.; Maddila, S. Microwave irradiated mild, rapid, one-pot and multicomponent synthesis of isoxazole-5(4H)-ones. Chem. Data Collect. 2021, 32, 100669.
- Kumar, T.S.; Robert, A.R.; Ganja, H.; Muralidhar, P.; Kerru, N.; Maddila, S. Purification free, chemoselective N-acylation of non-nucleophilic nitrogen heterocycles using oxyma and benzotriazole activations. Chem. Data Collect. 2021, 32, 100654.
- Kerru, N.; Maddila, S.N.; Maddila, S.; Sobhanapuram, S.; Jonnalagadda, S.B. Synthesis and antimicrobial activity of novel thienopyrimidine linked rhodanine derivatives. Can. J. Chem. 2019, 97, 94–99.
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Design of carbon carbon and carbon–heteroatom bond formation reactions under green conditions. Curr. Org. Chem. 2019, 23, 3156–3192.
- Immandhi, S.S.A.; Nagaraju, K.; Maddila, S.; Jonnalagadda, S.B. Recent progresses in the multicomponent synthesis of dihydropyridines by applying sustainable catalysts under green conditions. Front. Chem. 2021, 9, 800236.
- Maedeh, S.; Roghieh, M.; Ebrahim, B.; Mohammadi-Khanaposhtani, M.; Homa, A.; Mohammad, S.A.; Samanesadat, H.; Ebrahim, Z.; Somayeh, M.; Mohammad, A.F.; et al. Quinazolinone-dihydropyranopyran hybrids as new α-glucosidase inhibitors: Design, synthesis, enzymatic inhibition, docking study and prediction of pharmacokinetic. Bioorg. Chem. 2021, 109, 104703.
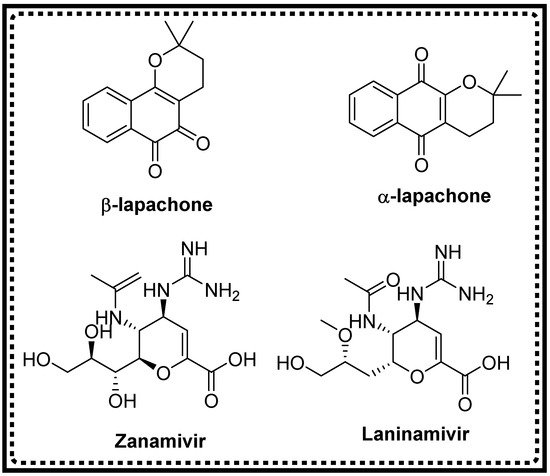
- Kumar, D.; Sharma, P.; Singh, H.; Nepali, K.; Gupta, G.K.; Jain, S.K.; Ntie-Kang, F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017, 59, 36977–36999.
- Ferreira, S.B.; da Silva, F.; Bezerra, F.A.; Lourenc, M.C.; Kaiser, C.R.; Pinto, A.C.; Ferreira, V.F. Synthesis of alpha-and beta-pyran naphthoquinones as a new class of antitubercular agents. Arch. Der Pharm. 2010, 343, 81–90.
- He, M.; Yang, N.; Sun, C.; Yao, X.; Yang, M. Modification and biological evaluation of novel 4-hydroxy-pyrone derivatives as non-peptidic HIV-1 protease inhibitors. Med. Chem. Res. 2011, 20, 200–209.
- Hussain, H.; Aziz, S.; Schulz, B.; Krohn, K. Synthesis of a 4H-anthra-pyran derivative and its antimicrobial activity. Nat. Prod. Commun. 2011, 6, 841–843.
- Sagar, S.; Thorata, B.; Kontham, R. Strategies for the synthesis of furo-pyranones and their application in the total synthesis of related natural products. Org. Chem. Front. 2021, 8, 2110.
- Bosica, G.; Abdilla, R. Recent advances in multicomponent reactions catalysed under operationally heterogeneous conditions. Catalysts 2022, 12, 725.
- Mamaghani, M.; Nia, R.H. A review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl. Aromat. Compd. 2021, 41, 223–291.
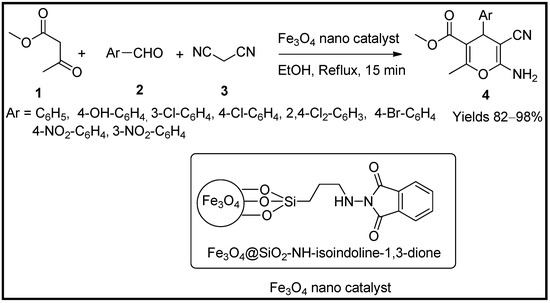
- Shabani, N.; Heravi, M.R.P.; Babazadeh, M.; Ghasemi, E.; Amini, M.; Robertson, C. 2-Aminoisoindoline-1,3-dione-functionalized Fe3O4/Chloro-silane core-shell nanoparticles as reusable catalyst: An efficient heterogeneous magnetic nanoparticles for synthesis of 4H-pyran derivatives through multicomponent reaction. Polycycl. Aromat. Compd. 2022, 42, 4561–4577.
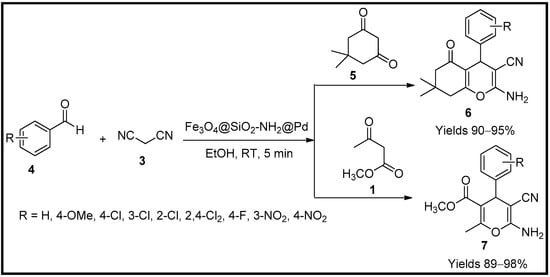
- Heravi, M.R.P.; Aghamohammadi, P.; Vessally, E. Green synthesis and antibacterial, antifungal activities of 4H-pyran, tetrahydro-4H-chromenes and spiro2-oxindole derivatives by highly efficient Fe3O4@SiO2@NH2@Pd(OCOCH3)2 nanocatalyst. J. Mol. Struct. 2022, 1249, 131534.
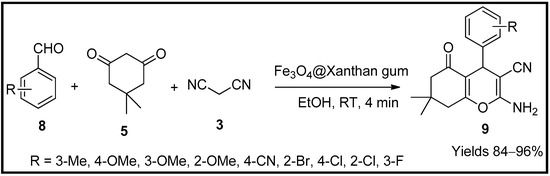
- Esmaeili, M.S.; Khodabakhshi, M.R.; Maleki, A.; Varzi, Z. Green, natural and low cost xanthum gum supported Fe3O4 as a robust biopolymer nanocatalyst for the one-pot synthesis of 2-amino-3-cyano-4H-pyran derivatives. Polycycl. Aromat. Compd. 2021, 41, 1953–1971.
- Maleki, B.; Reiser, O.; Esmaeilnezhad, E.; Choi, H.J. SO3H-dendrimer functionalised magnetic nanoparticles (Fe3O4@D-NH-(CH2)4-SO3H): Synthesis, characterisation and its application as a novel and heterogeneous catalyst for the one-pot synthesis of polyfunctionalised pyrans and polyhydroquinolines. Polyhedron 2019, 162, 129–141.
- Mohammadi, P.; Sheibani, H. Synthesis and characterisation of Fe3O4@SiO2 guanidine-poly acrylic acid nanocatalyst and using it for one-pot synthesis of 4H-benzopyrans and dihydropyranochromenes in water. Mater. Chem. Phys. 2019, 228, 140–146.
- Jamshidi, A.; Maleki, B.; Zonoz, F.M.; Tayebee, R. HPA-dendrimer functionalised magnetic nanoparticles (Fe3O4@DNH2-HPA) as a novel inorganic-organic hybrid and recyclable catalyst for the one-pot synthesis of highly substituted pyran derivatives. Mater. Chem. Phys. 2018, 209, 46e59.
- Elhamifar, D.; Ramazani, H.; Norouzi, M.; Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: A novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo-pyrans under ultrasonic conditions. J. Colloid Interface Sci. 2018, 511, 392–401.
- Aghbash, K.O.; Pesyan, N.N.; Batmani, H. Fe3O4@: A novel magnetically reusable nanostructured catalyst for clean in situ synthesis of substituted 2-aminodihydropyranopyran-3-cyano. Appl. Organomet. Chem. 2019, 33, e5227.
- Teimuri-Mofrad, R.; Gholamhosseini-Nazari, M.; Payami, E.; Esmati, S. Ferrocene-tagged ionic liquid stabilised on silica-coated magnetic nanoparticles: Efficient catalyst for the synthesis of 2-amino-3-cyano-4H-pyran derivatives under solvent-free conditions. Appl. Organomet. Chem. 2017, 32, e3955.
- Moghaddam-Manesh, M.; Ghazanfari, D.; Sheikhhosseini, E.; Akhgar, M. Synthesis of bioactive magnetic nanoparticles spirodithiine]@Ni (NO3)2 supported on Fe3O4@SiO2@CPS as reusable nanocatalyst for the synthesis of functionalised 3,4-dihydro-2H-pyran. Appl. Organomet. Chem. 2020, 34, e5543.
- Maleki, H.; Rakhtshah, J.; Shaabani, B. Effective one-pot synthesis of tetrahydrobenzopyran derivatives using nickel Schiff-base complex immobilised on iron oxide nanoparticles. Appl. Organomet. Chem. 2020, 34, e5683.
- Dazmiri, M.G.; Alinezhad, H.; Hossaini, Z.; Bekhradnia, A.R. Green synthesis of Fe3O4/ZnO magnetic core-shell nanoparticles by Petasites hybridus rhizome water extract and their application for the synthesis of pyran derivatives: Investigation of antioxidant and antimicrobial activity. Appl. Organomet. Chem. 2020, 34, e5731.
- Hassanzadeh-Afruzi, F.; Dogari, H.; Esmailzadeh, F.; Maleki, A. Magnetized melamine-modified polyacrylonitrile (/Fe3O4) organometallic nanomaterial: Preparation, characterisation, and application as a multifunctional catalyst in the synthesis of bioactive dihydropyrano pyrazole and 2-amino-3-cyano 4H-pyran derivatives. Appl. Organomet. Chem. 2021, 35, e6363.
- Heravi, M.M.; Beheshtiha1, S.Y.S.; Dehghani, M.; Hosseintash, N. Using magnetic nanoparticles Fe3O4 as a reusable catalyst for the synthesis of pyran and pyridine derivatives via one-pot multicomponent reaction. J. Iran. Chem. Soc. 2015, 12, 2075–2081.
- Sandaroos, R.; Nazif, A.; Molaei, H.; Salimi, S. Multi-component synthesis of a new series of 4H-furopyrans, with iron(III) triflate as catalyst. Res. Chem. Intermed. 2015, 41, 5033–5040.
