Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Despite the remarkable significance and encouraging breakthroughs of intracellular enzyme-instructed self-assembly of peptides (IEISAP) in disease diagnosis and treatment, a comprehensive review that focuses on this topic is still desirable.
- cancer theranostics
- bioimaging
- antibacterial
1. Introduction
Molecular self-assembly is a bottom-up, controllable, and effective way to produce functional ordered supramolecular materials with various dimensions from nanoscale to microscale, in which molecules spontaneously and hierarchically assemble by both internal and external interactions [1][2]. In nature, different biomolecules, such as biopolymers, peptides, proteins, and nucleic acids (DNA and RNA), can self-assemble into diverse nanostructures, inspiring the design, synthesis, and applications of self-assembled biomaterials in the fields of nanotechnology, environmental science, energy storage, biomedicine, and others [3][4][5][6]. Different stimuli can be harnessed to trigger molecular self-assembly in vitro or in vivo for obtaining desired materials [7][8][9][10], such as the building block concentration [11][12], enzyme [13], solvent [14], temperature [15], pH [16][17], ionic strength [17], metal ion [7], physical stimulation [8], and ligand–receptor interaction [9]. Recently, many research efforts have been devoted by a variety of researchers to the structural design, functional tailoring, and applications of enzyme-instructed self-assembly (EISA) of peptides, due to its following merits [10][13]: First, the structure of peptides can be modified easily by changing amino acid sequence. Second, the self-assembly of peptides into nanofibers, nanoparticles, nanoaggreates, and hydrogels can be tuned by controlling experimental conditions. Third, the peptide-derived materials have satisfactory bioactivity and biocompatibility.
Peptides consist of several or dozens of amino acids linked by amide bonds. They are ubiquitous in organisms and have critical biological functions. In addition, they are inherently bioactive, biocompatible, and biodegradable, making them ideal building units for functional biomaterials. There are twenty natural amino acids for peptide synthesis, which share the same backbone structure but vary in the R groups (side groups), enabling the construction of an enormous number of peptide sequences with diverse properties, suitable for use as assembling blocks. With specific design, peptides can have the ability to mimic the self-assembly behavior of proteins, further making them excellent choices for constructing materials with highly ordered structures and diverse functions. For instance, aromatic dipeptides [18], peptide-amphiphiles [19], and polypeptides [15] have been demonstrated to be molecular building blocks with excellent self-assembly properties, which can produce nanoscale structures, such as nanofibers and nanoparticles, through various noncovalent interactions between amino acid residues, such as ionic, hydrogen bonding, hydrophobic, and π–π stacking interactions. In addition to the self-assembling peptide units, self-assembled peptide-based precursors generally also consist of functional components and responsive/targeting groups. Peptides are easy to manipulate and synthetically accessible due to their simple linear structures. They can be either isolated from living organisms or synthesized through chemical methods, such as solid-phase peptide synthesis, ring-opening polymerization, and solution phase synthesis [20][21].
Enzymes have been considered as one of the most versatile strategies to control peptide self-assembly due to their reliable selectivity and mild reaction conditions in the catalysis of chemical reactions, as well as their good biocompatibility and spatiotemporality in cellular environments [10]. Since the expression and distribution of enzymes differ by the types and states of cells, tissues, and organs, when using an enzymatic reaction to realize the self-assembly of peptides into hierarchical nanamaterials, one can manipulate the delivery, function, and response of a nanomaterial according to a specific biological environment, therefore offering an accessible route to create desired materials for biomedical uses. In particular, intracellular enzymatic self-assembly of peptides provides a unique means for researchers to combine molecular self-assembly with intrinsic enzymatic reactions inside cells for developing novel biomaterials at the supramolecular level. In this context, different enzymes have been leveraged in manipulating peptide self-assembly in living cells, including alkaline phosphatase (ALP), caspase, furin, hyaluronidase (HAase), among others [10][22][23]. For instance, the most frequently used enzyme for intracellular enzyme-instructed self-assembly of peptides (IEISAP) is ALP. ALP is overexpressed on the cell membranes of tumor cells, such as Saos-2, HeLa, Hep G2, and MESSA/Dx5 [24][25][26][27], bacteria [28][29][30], and tear fluid [31]. ALP dephosphorylates the peptide-derived precursors, generating more hydrophobic products for peptide assembly/aggregation and realizing in situ self-assembly of peptides into supramolecular nanostructures in living systems.
Although traditional strategies that employ nanostructures assembled ex situ have demonstrated increased bioavailability and targetability due to the enhanced permeability and retention effect and the multivalent effect, the preassembled structures obtained from ex situ assembly sometimes suffer from inherent instability under sophisticated physiological environments in vivo. Exploiting the dynamic nature of molecular self-assembly, in situ assembly/reassembly has shown promising results in the prolonged retention of imaging and therapeutic agents, thus promoting its potential applications in long-term imaging and sustained therapeutic release. The main advantage of the IEISAP strategy is that the assembly can be precisely controlled to occur in the vicinity of a specific physiological or even pathological site by the fine tuning of peptide building units with targeted accumulation and responsive retention properties, leading to a low detection limit, high imaging quality, or great therapeutic efficacy [32]. The general processes for in vivo enzyme-activated self-assembly of peptides are described as follows: the peptide-derived precursors often contain a substrate motif, which will be recognized by a specific enzyme of interest. Upon arriving at the target cells, the precursors are converted by cellular enzymes into amphiphilic building blocks that self-assemble spontaneously into functional ordered supramolecular materials by noncovalent interactions (hydrophobic interaction, π−π interaction, hydrogen bonding, and electrostatic interaction), which can endow the peptides with improved stability, increased mechanical strength, or enhanced activity. In addition to noncovalent interactions, biocompatible condensation [33] and intracellular macrocyclization reaction [34] strategies have also been established to realize the desirable structure formation in living systems.
Since Bing Xu’s group first demonstrated the enzyme-instructed self-assembly of aromatic peptide amphiphiles within biological systems in 2007 [35], IEISAP has found many applications, due to the development of various peptides/peptide derivatives and the discovery of various enzymes that can be used for IEISAP. IEISAP allows for the creation of peptide nanostructures with diverse functions for high-performance biological applications, ranging from disease diagnosis to treatment, such as cancer tissue/cell imaging, enzyme activity assay, cancer therapy, and antibacterial application, and it has high potential in reducing drug toxicity, improving drug targeting, and enhancing drug delivery efficiency. However, a comprehensive review that specifically focuses on the use of IEISAP systems for biomedical applications is still lacking, although several reviews regarding self-assembled peptide-based materials (which do not specifically focus on the materials prepared via IEISAP) for biomedical applications have already been published [13][20][21][36][37][38]. Researchers will specifically focus on the developments of in situ enzyme-activated self-assembly of peptides for biomedical applications, including the imaging and treatment of diseases (mainly cancers) (Scheme 1). Researchers will illustrate the design, working mechanisms, and functions/applications of IEISAP. Finally, researchers will propose some current challenges and future research directions in this field.
Scheme 1. IEISAP for various imaging and disease treatment applications.
2. IEISAP for Imaging Applications
In terms of biomedical applications, bioimaging technologies are urgently calling for highly efficient probes/contrast agents for high-performance bioimaging, including molecular imaging, cell imaging, and tissue/organ imaging. IEISAP has been employed in improving the performance of routine modalities for bioimaging, such as fluorescence imaging, photoacoustic (PA) imaging, magnetic resonance imaging (MRI), positron-emission tomography (PET) imaging, computed tomography (CT) imaging, and multimodal imaging.
2.1. Fluorescence Imaging
The fluorescent IEISAP materials display varied structures and functionalities, and can be employed as potential fluorescent probes for high-performance biomedical imaging with prolonged tumor retention [39], high (photo)stability [40][41][42][43], and targeted biological distribution [44][45]. Generally, the building blocks for fluorescent IEISAP materials are peptide−fluorophore conjugates. The peptide units for self-assembly can be covalently linked with different fluorogens, such as 4-nitro-2,1,3-benzoxadiazole (NBD) [44][46][47][48][49][50], aggregation-induced emission luminogens (AIEgens) [45][51][52][53][54][55][56], the near-infrared (NIR) dye cyanine (Cy) [40][41][42], the fluorescent dye Alex Fluor 647 [39], the coumarin dye [57], 1,8-naphthalic anhydride [58], and fluorescein isothiocyanate (FITC) [43], to generate peptide−fluorophore conjugates as the building blocks for IEISAP. Specific enzymes in vivo and/or in cells can trigger the self-assembly of these peptide−fluorophore conjugates into nanostructures such as nanofibers [41][43][44][46][47][48][50][55][56][57][58] and nanoparticles [42][53] in which the fluorescence can be enhanced/decreased. These enzymes include ALP [43][46][50][51][55][57][58][59][60], caspase-3/7 [40][42][52][53], cathepsin B [45][54], matrix metalloproteinases (MMPs) [39][41], esterase [47], enterokinase (ENTK) [48], sirtuin family, which consists of seven isoform 5 (SIRT5) [44], and autophagy-related 4 homolog B (ATG4B) [56]. IEISAP has been utilized to monitor enzyme activity [42][43][44][50][55][57], self-assembly of peptides [46][60], subcellular compartments [44][48], apoptosis [52][53], and autophagy [56] in living cells, as well as visualize tumor tissues and cells [39][40][41][43][45][51][53][54].
For instance, Yang et al. reported SIRT5-mediated self-assembly of fluorescent peptide precursors for imaging the SIRT5 activity in mitochondria [44]. SIRT5, a mitochondria-oriented enzyme, belongs to a family of nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases, and is closely associated with the regulation of diverse biological processes, such as apoptosis, fatty acid metabolism, and reactive oxygen defense. Nevertheless, designing biosensors for detecting intracellular SIRT5 activity is challenging, yet lacking. The peptide precursor consisted of an environment-sensitive fluorophore NBD for imaging, a phenylalanine-rich peptide fragment, and a Ksucc (succinylated lysine) switch module (Figure 1a). These amphipathic peptide precursors with low molecular weight could effectively enter cells, in which their aggregation in the cellular environment enhanced their cell internalization. Upon arriving at mitochondria, the negatively charged Ksucc in the peptide precursor was desuccinylated by SIRT5 to generate the positively charged lysine residue, forming a desuccinylated peptide building block with zero charge (Figure 1a). The unique zwitterionic nature of the peptide building block increased the electrostatic interaction between each other, leading to the self assembly into nanofibers. Meanwhile, the NBD in the nanofibers produced bright fluorescence due to the hydrophobic environment in nanofibers, realizing fluorescence detection or imaging of cellular SIRT5 activity (Figure 1b). In addition to bioimaging applications, fluorescent IEISAP materials have also been applied in cancer theranostics [40][45][51][53][54].
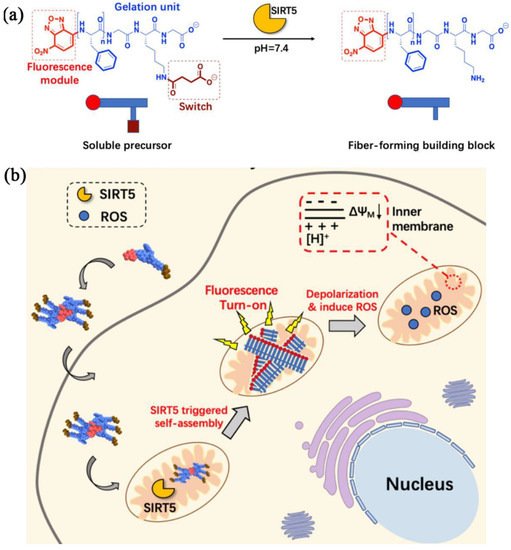
Figure 1. IEISAP for fluorescence imaging. (a) Molecular structures of the soluble precursor and the fiber-forming building block. The soluble precursor can be desuccinylated by SIRT5 to generate the fiber-forming building block. (b) Scheme illustrating the intracellular fiber formation in mitochondria via the specific interaction of the internalized peptide precursors with the SIRT5 enzyme, as well as the effect of the formation of nanofibers on the depolarization of mitochondrial membrane potential and promotion of ROS generation. Reprinted/adapted with permission from [44]. Copyright 2020, American Chemical Society.
2.2. Photoacoustic (PA) Imaging
Photoacoustic (PA) imaging is a noninvasive imaging method by detecting ultrasonic waves produced from the transient thermoelastic expansion of biological tissues upon light absorption from a pulsed laser. PA imaging simultaneously possesses the sensitivity of fluorescence imaging, and the high spatial resolution (up to tens of micrometers) and deep tissue penetration (up to a few centimeters) of ultrasound imaging, holding great promise for the accurate evaluation of important physiological and pathological processes. Nevertheless, there are only a few naturally occurring light absorbers, such as hemoglobin and melanin. Thus, various exogenous photoacoustic agents, based on materials including IEISAP assemblies, have been developed to enhance the photoacoustic contrast. The photoacoustic agents based on IEISAP materials that can respond to various enzymes, such as gelatinase [61][62], autophagy-specific enzyme ATG4B [63], ALP [64], caspase-1 [65], caspase-3 [66], and furin [67], have been utilized for imaging tumors [62][63][64][66][67] and detecting bacteria [32][65]. In addition to enhanced photoacoustic signals, the IEISAP strategy endows photoacoustic agents with high stability in vivo [32][67], desirable targeting properties [65][67], and long retention in tumor sties [62][66]. Wang et al. developed a caspase-3-activatable PA imaging probe (termed 1-RGD) for real-time and high-resolution imaging of tumor apoptosis. 1-RGD contained a tumor-targeting cyclic peptide (cyclic Arg-Gly-Asp, c-RGD), 2-cyano-6-hydroxyquinoline (CHQ), a D-cysteine (D-Cys) residue, a caspase-3-cleavable peptide substrate (Asp-Glu-Val-Asp, DEVD), a glutathione (GSH)-reducible disulfide bond, and a clinically used NIR dye (indocyanine green, ICG) (Figure 2a) [66]. In apoptotic tumors, intracellular GSH and active caspase-3 uncaged the thiol and amino groups of the D-Cys residue in 1-RGD, respectively (Figure 2b). The free d-Cys could interact with CHQ by fast intramolecular condensation to produce a cyclized product 1-cycl that was more hydrophobic and rigid than 1-RGD, causing stronger intermolecular interactions, such as hydrophobic interaction and π–π stacking, to enhance the molecular self-assembly into nanoparticles. Compared with 1-RGD, the density of ICG molecules in the nanoparticles was higher, leading to the lower NIR fluorescence because of the aggregation-caused quenching (ACQ) effect and increased PA signals, due to the augmented nonradiative relaxation processes (Figure 2c). Meanwhile, the larger size of the nanoparticles, compared with that of 1-RGD, endowed them with prolonged retention in apoptotic tumor regions. Collectively, confined PA signal improvement in apoptotic tumor tissues was realized to monitor caspase-3 activity for evaluating the apoptosis status in the whole tumor tissue, facilitating early and real-time evaluation of tumor therapeutic efficacy, prior to the alteration in tumor size.
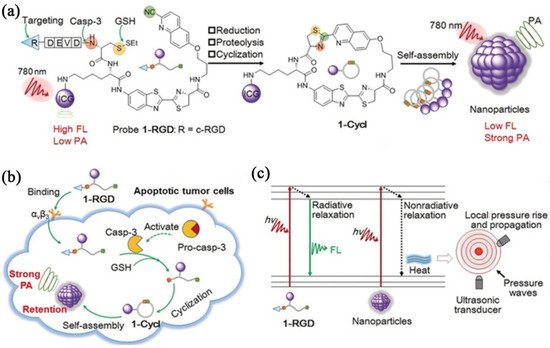
Figure 2. IEISAP for PA imaging. (a) Chemical structure and proposed chemical conversion of 1-RGD to 1-cycl, as well as the self-assembly of 1-cycl to nanoparticles. (b) Scheme depicting the mechanism of using 1-RGD for PA imaging of caspase-3 activity in apoptotic tumor cells. (c) Jablonski diagram of the proposed mechanism to amplify PA signal by augmenting nonradiative relaxation of the excited ICG fluorophores within nanoparticles. Reprinted/adapted with permission from [66]. Copyright 2019, John Wiley & Sons, Inc.
Besides tumor diagnosis, PA imaging based on IEISAP has been employed for bacterial infection diagnosis [32][65]. Currently, the available effective approaches for early-stage pathogen diagnosis cannot be applied in vivo and suffer from low sensitivity and specificity [32]. Therefore, new strategies are urgently needed to be developed for accurately visualizing bacterial infections in situ. Li et al. developed a photoacoustic contrast agent, assembled from an enzyme-responsive peptide, for in vivo specific and sensitive imaging of bacterial infection [32]. The building block (Ppa-PLGVRG-Van) contained pyropheophorbide-α (Ppa) as a signal ligand, Pro-Leu-Gly-Val-Arg-Gly (PLGVRG) as an enzyme-activatable peptide linker, and vancomycin (Van) as a targeting molecule. Ppa-PLGVRG-Van could be selectively anchored to the Gram-positive bacterial cell wall through multiple hydrogen bonding interactions between Van and the terminal D-alanyl-D-alanine moieties of the N-acetyl muramic acid (NAM)/N-acetyl-glucosamine (NAG) peptides on the cell wall of Gram-positive bacteria. Then, the PLGVRG linker was specifically cleaved by gelatinase in gelatinase-overexpressing bacteria to produce Ppa-PLG. With enhanced hydrophobicity and decreased steric hindrance, Ppa-PLG self-assembled into twisted fibers, resulting in an increased heat conversion efficiency and an enhanced photoacoustic signal. Accordingly, Ppa-PLGVRG-Van could successfully image bacterial infections with high sensitivity and specificity. Moreover, this contrast agent could discriminate bacterial infection from sterile inflammation in vivo, and could also detect Gram-positive, gelatinase-expressing bacteria with high sensitivity. The PA contrast agents based on IEISAP offer new possibilities for the specific and sensitive diagnosis of bacterial infections in vivo.
2.3. Magnetic Resonance Imaging (MRI)
MRI is a widely used, powerful, and noninvasive imaging method for clinical diagnosis of tumors with unlimited penetration depth and high spatial resolution. Nevertheless, the sensitivity of MRI is low, requiring constant utilization of contrast agents (CAs) to promote the imaging quality and accuracy by altering the spin–lattice relaxation time (T1) or spin–spin relaxation time (T2). To solve this issue, IEISAP has been harnessed to increase the relaxation efficiency of traditional T1 CAs, such as Gd(III)-based CAs [68][69][70], and T2 CAs, such as superparamagnetic iron oxide (SPIO) nanoparticle-based CAs [71][72], for tumor imaging with the help of enzymes that are overexpressed in cancer cells, such as ALP [68][73], caspase 3/7 (Casp3/7) [70][71], MMP-2 [69], and furin [72]. For example, Ding et al. functionalized Fe3O4 nanoparticles (IONPs) with a dual-functional fluorine probe 4-(trifluoromethyl)benzoic acid (TFMB)-Arg-Val-Arg-Arg-Cys(StBu)-Lys-CBT to obtain IONP@1 (Figure 3) [72]. The as-synthesized IONP@1 was composed of the TFMB-Arg-Val-Arg-Arg (TFMB-RVRR) substrate for furin cleavage and providing 19F nuclear magnetic resonance (NMR)/MRI signal, a 2-cyanobenzothiazole (CBT) residue linked with a caged cysteine moiety for click condensation reaction, and Fe3O4 NP (IONP) as a T2 MRI CA. When IONP@1 encountered furin-overexpressing cells, the intracellular GSH reduction of the disulfide bonds in IONP@1 and furin-mediated cleavage of TFMB-RVRR resulted in the generation of 1,2-aminothiol groups. The crosslinking of IONPs was achieved by the click condensation reaction between the 1,2-aminothiol groups and cyano moieties, forming IONP aggregates. The formation of IONP aggregates caused a lower T2 value of the surrounding water protons, and consequently a stronger T2 magnetic resonance signal. Meanwhile, the peeling-off of TFMB-RVRR from IONP relieved the paramagnetic relaxation enhancement effect, thereby turning “on” the 19F NMR/MRI signal. In this way, IONP@1 was successfully employed for precise dual-mode (1H and 19F) MRI of tumors in zebrafish under 14.1 T. Researchers proposes a solution to address the dilemma between selectivity and sensitivity of traditional MRI sensors.
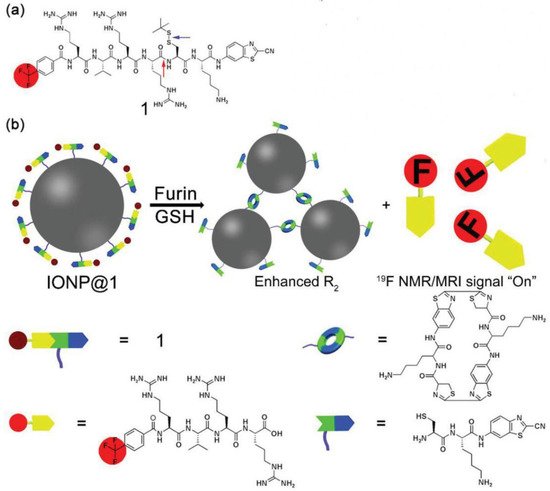
Figure 3. IEISAP for MRI. (a) Structural diagram of compound 1. The red arrow indicates the furin-mediated cleavage site, while the blue arrow points to the GSH reduction site. (b) Schematic demonstration of intracellular furin/GSH-regulated generation of IONP aggregates from IONP@1 for improved transverse relaxation rate (R2) and release of TFMB-Arg-Val-Arg-Arg-OH residues for turning on the 19F NMR/MRI signal. Reprinted/adapted with permission from [72]. Copyright 2019, John Wiley & Sons, Inc.
2.4. Positron-Emission Tomography (PET) Imaging
PET is a routine method of tumor imaging in clinic, offering valuable information to discriminate changes in cancer at the cellular level by utilizing radiotracers to image biological processes with ultrahigh sensitivity. However, few tumor-targeted PET imaging probes are available for precisely visualizing a specific tumor, urgently requiring the development of tumor-targeted PET imaging probes to increase the specificity of PET imaging. To this end, IEISAP has been implemented to develop tumor-targeting radioactive probes for enhanced microPET imaging of tumors [74][75]. As an example, Wang et al. rationally developed a furin-responsive radiotracer Acetyl-Arg-Val-Arg-Arg-Cys(StBu)-Lys-(DOTA-68Ga)-CBT (CBT-68Ga; DOTA is the abbreviation of “1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid”) and coinjected it with its cold analogue CBT-Ga in furin-overexpressing MDA-MB-468 cancer cells, leading to the formation of 68Ga nanoparticles (CBT-68Ga-NPs) [75]. The formation of CBT-68Ga-NPs notably improved microPET imaging performance of the tumor in vivo. In brief, in cancer cells overexpressing furin, disulfide bond reduction and furin cleavage of Arg-Val-Arg-Arg (RVRR) occurred, converting CBT-Ga to the active intermediate Cys-Lys(DOTA-Ga)-CBT. Cys-Lys(DOTA-Ga)-CBT immediately underwent a CBT–Cys condensation reaction to generate the cyclized oligomers (CBT-Ga-Dimer and CBT-Ga-Trimer) that self-assembled into nanoparticles (CBT-Ga-NPs) with an average diameter of 258.3 nm under the physiological condition. In vivo microPET imaging data showed that the mice coadministrated with CBT-68Ga and CBT-Ga had a tumor/liver ratio 9.1-fold of that of the mice administrated with CBT-68Ga, realizing improved tumor microPET imaging. The presence of the cold analogue CBT-Ga was needed for the intracellular formation of CBT-68Ga-NPs by overcoming the interference of intracellular Cys with the CBT–Cys condensation of the low-concentration CBT-68Ga in vivo. It successfully applies IEISAP to improve the microPET imaging performance. It is also expected that IEISAP will offer a strategy to devise a variety of radioactive agents for more sensitive and precise microPET imaging of tumors in the future.
2.5. Computed Tomography (CT)
X-ray computed tomography (CT) images body structures and tissues by utilizing the different absorption effects from different human tissues with the assistance of contrast agents. As a convenient and efficient diagnostic method, CT imaging has become a good alternative to the body anatomization technique [76]. IEISAP has been applied to endow the CT contrast agents with tumor-targeting ability [77][78][79] and long tumor retention effect [78][79], such as gold nanoparticles (AuNPs) [77][79] and 89Zr [78]. For instance, Sun et al. reported a tumor-specific AuNP-based nanoprobe based on IEISAP for dual CT/optical imaging of cancer [77]. AuNPs were first linked with glycol chitosan (GC) polymers to produce physiologically stable and tumor-targeting GC-AuNPs. GC-AuNPs were further chemically conjugated with an MMP-responsive fluorescent unit (Cy5.5-Gly-Pro-Leu-Gly-Val-Arg-Gly-Lys(BHQ)-Gly-Gly), which was obtained by coupling the NIR fluorescent dye (Cy5.5) and black hole quencher (BHQ) to both ends of the MMP cleavable peptide, resulting in MMP-GC-AuNPs. In MMP-GC-AuNPs, the fluorescence of Cy5.5 was strongly quenched, due to the combinational quenching effect from the gold particle surface and the organic BHQ. The quenched fluorescence of Cy5.5 was recovered when the peptides were cleaved by MMPs that were overexpressed in the tumor tissue. The authors demonstrated that MMP-GC-AuNPs could effectively accumulate in the tumor tissue, visualizing the tumor tissue using CT with great spatial resolution and optical imaging with excellent sensitivity simultaneously in the tumor-bearing mouse model, which offered not only accurate tumor anatomical information, but also MMP-dependent biological information.
2.6. Dual/Multimodal Imaging
Dual/multimodal imaging technologies consist of at least two imaging functions combined in one imaging probe, and they are required for advancing biomedical and clinical research. Ideal dual/multimodal imaging approaches should be synergistically combined to overcome the weak points of each imaging method, offering distinct imaging information. Controlling the self-assembly process to simultaneously activate dual/multimodal imaging signals in a small-molecule probe is challenging. IEISAP has been successfully deployed to develop activatable dual/multimodal probes for in vivo imaging of tumor and enzyme activity in real time, including dual CT/optical imaging [77], multimodal fluorescence/PET/CT imaging [78], and dual NIR fluorescence/MR imaging [80]. For instance, Yan et al. rationally designed and fabricated an activatable bimodal probe (P-CyFF-Gd) for molecular imaging using NIR fluorescence and MR by combining a fluorogenic reaction with enzyme-responsive in situ self-assembly [80]. P-CyFF-Gd was composed of a prequenched NIR fluorophore (merocyanine, Cy-Cl), linked with a phosphate group (−PO3H) that could be recognized by ALP, a paramagnetic DOTA-Gd chelate for MRI, and a hydrophobic dipeptide Phe-Phe (FF) linker to promote self-assembly (Figure 4a). P-CyFF-Gd was water-soluble due to the presence of the hydrophilic −PO3H and DOTA-Gd ligands, and it displayed quenched NIR fluorescence and low r1 relaxivity. Upon systemic administration, P-CyFF-Gd could easily extravasate and deeply diffuse into tumor tissues because of its hydrophilicity and small molecular size (Figure 4b). In tumor tissues that contain ALP, ALP dephosphorylated P-CyFF-Gd to produce hydrophobic CyFF-Gd, emitting NIR fluorescence at 710 nm. Meanwhile, the FF dipeptide of CyFF-Gd provided effective intermolecular interactions to induce molecular self-assembly, resulting in the formation of fluorescent and magnetic NPs. The assembled NPs possessed a significantly larger molecular size than P-CyFF-Gd, which likely reduced molecular rotation and increased tumbling time (τR) of Gd-chelates, thus increasing r1 relaxivity. Furthermore, the NPs were also prone to bind to the plasma membrane, which could facilitate their cellular uptake and lysosomal localization via endocytosis. The prolonged retention of the NPs in ALP-expressing tumors could be realized, whereas residual P-CyFF-Gd was likely washed away from ALP-negative normal tissues to avoid side effects. Therefore, the formation of NPs simultaneously achieved the enhancements of the NIR fluorescence at 710 nm (>70 folds) and the r1 relaxivity (~2.3-fold), allowing the real-time detection of ALP activity in live tumor cells and mice with high sensitivity and high spatial resolution. Similar strategies can be adopted to construct other enzyme-activatable bimodal sensors for in-situ and real-time tracking of enzyme activity and location.
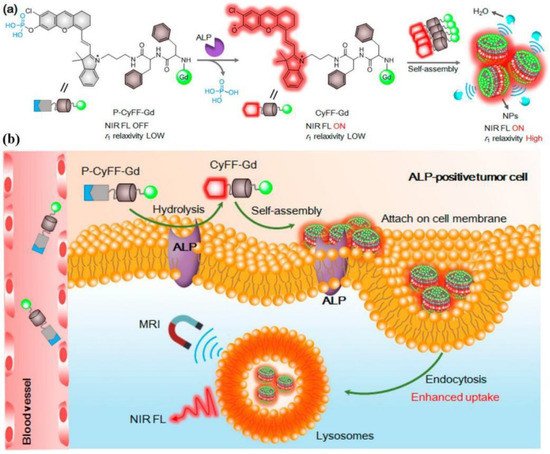
Figure 4. Schematic description of an ALP-activatable NIR fluorescence (FL)/MR bimodal probe for in vivo imaging. (a) Chemical structure of P-CyFF-Gd, the ALP-regulated fluorogenic reaction of P-CyFF-Gd, and in situ self-assembly of CyFF-Gd into NPs that displayed enhanced NIR FL and r1 relaxivity. (b) Proposed mechanism of P-CyFF-Gd for NIR FL/MR bimodal imaging of ALP-expressing tumor cells in vivo. Reprinted/adapted with permission from [80]. Copyright 2019, American Chemical Society.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196557
References
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421.
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95.
- Wang, L.; Sun, Y.; Li, Z.; Wu, A.; Wei, G. Bottom-up synthesis and sensor applications of biomimetic nanostructures. Materials 2016, 9, 53.
- Helbing, C.; Deckert-Gaudig, T.; Firkowska-Boden, I.; Wei, G.; Deckert, V.; Jandt, K.D. Protein handshake on the nanoscale: How albumin and hemoglobin self-assemble into nanohybrid fibers. ACS Nano 2018, 12, 1211–1219.
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595.
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182.
- Zou, R.; Wang, Q.; Wu, J.; Wu, J.; Schmuck, C.; Tian, H. Peptide self-assembly triggered by metal ions. Chem. Soc. Rev. 2015, 44, 5200–5219.
- Liu, F.H.; Cong, Y.; Qi, G.B.; Ji, L.; Qiao, Z.Y.; Wang, H. Near-infrared laser-driven in situ self-assembly as a general strategy for deep tumor therapy. Nano Lett. 2018, 18, 6577–6584.
- Haburcak, R.; Shi, J.; Du, X.; Yuan, D.; Xu, B. Ligand–receptor interaction modulates the energy landscape of enzyme-instructed self-assembly of small molecules. J. Am. Chem. Soc. 2016, 138, 15397–15404.
- Kim, B.J. Enzyme-instructed self-assembly of peptides: From concept to representative applications. Chem. Asian J. 2022, 17, e202200094.
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26.
- Lu, X.; Li, X.; Guo, K.; Xie, T.Z.; Moorefield, C.N.; Wesdemiotis, C.; Newkome, G.R. Probing a hidden world of molecular self-assembly: Concentration-dependent, three-dimensional supramolecular interconversions. J. Am. Chem. Soc. 2014, 136, 18149–18155.
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides. Adv. Mater. 2020, 32, 1805798.
- Rehm, T.H.; Schmuck, C. Ion-pair induced self-assembly in aqueous solvents. Chem. Soc. Rev. 2010, 39, 3597–3611.
- Dreher, M.R.; Simnick, A.J.; Fischer, K.; Smith, R.J.; Patel, A.; Schmidt, M.; Chilkoti, A. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J. Am. Chem. Soc. 2008, 130, 687–694.
- Ghosh, A.; Haverick, M.; Stump, K.; Yang, X.; Tweedle, M.F.; Goldberger, J.E. Fine-tuning the pH trigger of self-assembly. J. Am. Chem. Soc. 2012, 134, 3647–3650.
- Zhou, D.; Dong, S.; Kuchel, R.P.; Perrier, S.; Zetterlund, P.B. Polymerization induced self-assembly: Tuning of morphology using ionic strength and pH. Polym. Chem. 2017, 8, 3082–3089.
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890.
- Kokkoli, E.; Mardilovich, A.; Wedekind, A.; Rexeisen, E.L.; Garg, A.; Craig, J.A. Self-assembly and applications of biomimetic and bioactive peptide-amphiphiles. Soft Matter 2006, 2, 1015–1024.
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, 1703444.
- Ren, C.; Wang, Z.; Wang, Q.; Yang, C.; Liu, J. Self-assembled peptide-based nanoprobes for disease theranostics and disease-related molecular imaging. Small Methods 2020, 4, 1900403.
- Miao, Q.; Pu, K. Emerging designs of activatable photoacoustic probes for molecular imaging. Bioconjugate Chem. 2016, 27, 2808–2823.
- Yang, Z.; Liang, G.; Xu, B. Enzymatic control of the self-assembly of small molecules: A new way to generate supramolecular hydrogels. Soft Matter 2007, 3, 515–520.
- Zhou, J.; Du, X.; Xu, B. Regulating the rate of molecular self-assembly for targeting cancer cells. Angew. Chem. Int. Ed. 2016, 55, 5770–5775.
- Zhang, H.; Ju, Q.; Pang, S.; Wei, N.; Zhang, Y. Recent progress of fluorescent probes for the detection of alkaline phosphatase (ALP): A review. Dyes Pigm. 2021, 194, 109569.
- Zhan, J.; Cai, Y.; He, S.; Wang, L.; Yang, Z. Tandem molecular self-assembly in liver cancer cells. Angew. Chem. Int. Ed. 2018, 57, 1813–1816.
- Zheng, Z.; Chen, P.; Xie, M.; Wu, C.; Luo, Y.; Wang, W.; Jiang, J.; Liang, G. Cell environment-differentiated self-assembly of nanofibers. J. Am. Chem. Soc. 2016, 138, 11128–11131.
- Yang, Z.; Liang, G.; Guo, Z.; Guo, Z.; Xu, B. Intracellular hydrogelation of small molecules inhibits bacterial growth. Angew. Chem. Int. Ed. 2007, 46, 8216–8219.
- Ren, C.; Wang, H.; Zhang, X.; Ding, D.; Wang, L.; Yang, Z. Interfacial self-assembly leads to formation of fluorescent nanoparticles for simultaneous bacterial detection and inhibition. Chem. Commun. 2014, 50, 3473–3475.
- Hughes, M.; Debnath, S.; Knapp, C.W.; Ulijn, R.V. Antimicrobial properties of enzymatically triggered self-assembling aromatic peptide amphiphiles. Biomater. Sci. 2013, 1, 1138–1142.
- Hu, Y.; Wang, Y.; Deng, J.; Ding, X.; Lin, D.; Shi, H.; Chen, L.; Lin, D.; Wang, Y.; Vakal, S.; et al. Enzyme-instructed self-assembly of peptide-drug conjugates in tear fluids for ocular drug delivery. J. Control. Release 2022, 344, 261–271.
- Li, L.L.; Ma, H.L.; Qi, G.B.; Zhang, D.; Yu, F.; Hu, Z.; Wang, H. Pathological-condition-driven construction of supramolecular nanoassemblies for bacterial infection detection. Adv. Mater. 2016, 28, 254–262.
- Liang, G.; Ren, H.; Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nat. Chem. 2010, 2, 54–60.
- Ye, D.; Liang, G.; Ma, M.L.; Rao, J. Controlling intracellular macrocyclization for the imaging of protease activity. Angew. Chem. Int. Ed. 2011, 50, 2275–2279.
- Yang, Z.; Liang, G.; Ma, M.; Gao, Y.; Xu, B. In vitro and in vivo enzymatic formation of supramolecular hydrogels based on self-assembled nanofibers of a β-amino acid derivative. Small 2007, 3, 558–562.
- Li, L.L.; Qiao, Z.Y.; Wang, L.; Wang, H. Programmable construction of peptide-based materials in living subjects: From modular design and morphological control to theranostics. Adv. Mater. 2019, 31, 1804971.
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv. Mater. 2017, 29, 1605021.
- Shy, A.N.; Kim, B.J.; Xu, B. Enzymatic noncovalent synthesis of supramolecular soft matter for biomedical applications. Matter 2019, 1, 1127–1147.
- Chien, M.P.; Carlini, A.S.; Hu, D.; Barback, C.V.; Rush, A.M.; Hall, D.J.; Orr, G.; Gianneschi, N.C. Enzyme-directed assembly of nanoparticles in tumors monitored by in vivo whole animal imaging and ex vivo super-resolution fluorescence imaging. J. Am. Chem. Soc. 2013, 135, 18710–18713.
- Zheng, R.; Yang, J.; Mamuti, M.; Hou, D.Y.; An, H.W.; Zhao, Y.; Wang, H. Controllable self-assembly of peptide-cyanine conjugates in vivo as fine-tunable theranostics. Angew. Chem. Int. Ed. 2021, 60, 7809–7819.
- An, H.W.; Hou, D.; Zheng, R.; Wang, M.D.; Zeng, X.Z.; Xiao, W.Y.; Yan, T.D.; Wang, J.Q.; Zhao, C.H.; Cheng, L.M.; et al. A near-infrared peptide probe with tumor-specific excretion-retarded effect for image-guided surgery of renal cell carcinoma. ACS Nano 2020, 14, 927–936.
- Ye, D.; Shuhendler, A.J.; Cui, L.; Tong, L.; Tee, S.S.; Tikhomirov, G.; Felsher, D.W.; Rao, J. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat. Chem. 2014, 6, 519–526.
- Dong, L.; Miao, Q.; Hai, Z.; Yuan, Y.; Liang, G. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015, 87, 6475–6478.
- Yang, L.; Peltier, R.; Zhang, M.; Song, D.; Huang, H.; Chen, G.; Chen, Y.; Zhou, F.; Hao, Q.; Bian, L.; et al. Desuccinylation-triggered peptide self-assembly: Live cell imaging of SIRT5 activity and mitochondrial activity modulation. J. Am. Chem. Soc. 2020, 142, 18150–18159.
- Han, H.; Jin, Q.; Wang, Y.; Chen, Y.; Ji, J. The rational design of a gemcitabine prodrug with AIE-based intracellular light-up characteristics for selective suppression of pancreatic cancer cells. Chem. Commun. 2015, 51, 17435–17438.
- Gao, Y.; Shi, J.; Yuan, D.; Xu, B. Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nat. Commun. 2012, 3, 1033.
- Zhou, J.; Du, X.; Li, J.; Yamagata, N.; Xu, B. Taurine boosts cellular uptake of small D-peptides for enzyme-instructed intracellular molecular self-assembly. J. Am. Chem. Soc. 2015, 137, 10040–10043.
- He, H.; Wang, J.; Wang, H.; Zhou, N.; Yang, D.; Green, D.R.; Xu, B. Enzymatic cleavage of branched peptides for targeting mitochondria. J. Am. Chem. Soc. 2018, 140, 1215–1218.
- Wang, H.; Feng, Z.; Del Signore, S.J.; Rodal, A.A.; Xu, B. Active probes for imaging membrane dynamics of live cells with high spatial and temporal resolution over extended time scales and areas. J. Am. Chem. Soc. 2018, 140, 3505–3509.
- Zhou, J.; Du, X.; Berciu, C.; He, H.; Shi, J.; Nicastro, D.; Xu, B. Enzyme-instructed self-assembly for spatiotemporal profiling of the activities of alkaline phosphatases on live cells. Chem 2016, 1, 246–263.
- Ji, S.; Gao, H.; Mu, W.; Ni, X.; Yi, X.; Shen, J.; Liu, Q.; Bao, P.; Ding, D. Enzyme-instructed self-assembly leads to the activation of optical properties for selective fluorescence detection and photodynamic ablation of cancer cells. J. Mater. Chem. B 2018, 6, 2566–2573.
- Shi, H.; Kwok, R.T.K.; Liu, J.; Xing, B.; Tang, B.Z.; Liu, B. Real-time monitoring of cell apoptosis and drug screening using fluorescent light-up probe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981.
- Yuan, Y.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Targeted theranostic platinum(IV) prodrug with a built-in aggregation-induced emission light-up apoptosis sensor for noninvasive early evaluation of its therapeutic responses in situ. J. Am. Chem. Soc. 2014, 136, 2546–2554.
- Yuan, Y.; Zhang, C.J.; Gao, M.; Zhang, R.; Tang, B.Z.; Liu, B. Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew. Chem. Int. Ed. 2015, 54, 1780–1786.
- Zhang, X.; Ren, C.; Hu, F.; Gao, Y.; Wang, Z.; Li, H.; Liu, J.; Liu, B.; Yang, C. Detection of bacterial alkaline phosphatase activity by enzymatic in situ self-assembly of the AIEgen-peptide conjugate. Anal. Chem. 2020, 92, 5185–5190.
- Lin, Y.X.; Qiao, S.L.; Wang, Y.; Zhang, R.X.; An, H.W.; Ma, Y.; Rajapaksha, R.P.Y.J.; Qiao, Z.Y.; Wang, L.; Wang, H. An in situ intracellular self-assembly strategy for quantitatively and temporally monitoring autophagy. ACS Nano 2017, 11, 1826–1839.
- Zhong, Y.; Zhan, J.; Xu, G.; Chen, Y.; Qin, Q.; Liao, X.; Ma, S.; Yang, Z.; Cai, Y. Enzyme-instructed self-assembly enabled monomer–excimer transition to construct higher ordered luminescent supramolecular assembly for activity-based bioimaging. Angew. Chem. Int. Ed. 2021, 60, 8121–8129.
- Zhang, Y.; Ding, Y.; Li, X.; Zhang, Z.; Zhang, X.; Chen, Y.; Yang, Z.; Shi, Y.; Hu, Z.W. Enzyme-instructed self-assembly enabled fluorescence light-up for alkaline phosphatase detection. Talanta 2022, 239, 123078.
- Liang, J.; Kwok, R.T.K.; Shi, H.; Tang, B.Z.; Liu, B. Fluorescent light-up probe with aggregation-induced emission characteristics for alkaline phosphatase sensing and activity study. ACS Appl. Mater. Interfaces 2013, 5, 8784–8789.
- Gao, Y.; Berciu, C.; Kuang, Y.; Shi, J.; Nicastro, D.; Xu, B. Probing nanoscale self-assembly of nonfluorescent small molecules inside live mammalian cells. ACS Nano 2013, 7, 9055–9063.
- Wang, L.; Yang, P.P.; Zhao, X.X.; Wang, H. Self-assembled nanomaterials for photoacoustic imaging. Nanoscale 2016, 8, 2488–2509.
- Zhang, D.; Qi, G.B.; Zhao, Y.X.; Qiao, S.L.; Yang, C.; Wang, H. In situ formation of nanofibers from purpurin18-peptide conjugates and the assembly induced retention effect in tumor sites. Adv. Mater. 2015, 27, 6125–6130.
- Lin, Y.X.; Wang, Y.; Qiao, S.L.; An, H.W.; Wang, J.; Ma, Y.; Wang, L.; Wang, H. “In vivo self-assembled” nanoprobes for optimizing autophagy-mediated chemotherapy. Biomaterials 2017, 141, 199–209.
- Wu, C.; Zhang, R.; Du, W.; Cheng, L.; Liang, G. Alkaline Phosphatase-triggered self-assembly of near-infrared nanoparticles for the enhanced photoacoustic imaging of tumors. Nano Lett. 2018, 18, 7749–7754.
- Cai, Q.; Fei, Y.; Hu, L.; Huang, Z.; Li, L.L.; Wang, H. Chemotaxis-instructed intracellular Staphylococcus aureus infection detection by a targeting and self-assembly signal-enhanced photoacoustic probe. Nano Lett. 2018, 18, 6229–6236.
- Wang, Y.; Hu, X.; Weng, J.; Li, J.; Fan, Q.; Zhang, Y.; Ye, D. A photoacoustic probe for the imaging of tumor apoptosis by caspase-mediated macrocyclization and self-assembly. Angew. Chem. Int. Ed. 2019, 58, 4886–4890.
- Dragulescu-Andrasi, A.; Kothapalli, S.R.; Tikhomirov, G.A.; Rao, J.; Gambhir, S.S. Activatable oligomerizable imaging agents for photoacoustic imaging of furin-like activity in living subjects. J. Am. Chem. Soc. 2013, 135, 11015–11022.
- Dong, L.; Qian, J.; Hai, Z.; Xu, J.; Du, W.; Zhong, K.; Liang, G. Alkaline phosphatase-instructed self-assembly of gadolinium nanofibers for enhanced T2-weighted magnetic resonance imaging of tumor. Anal. Chem. 2017, 89, 6922–6925.
- Zhang, J.; Mu, Y.L.; Ma, Z.Y.; Han, K.; Han, H.Y. Tumor-triggered transformation of chimeric peptide for dual-stage-amplified magnetic resonance imaging and precise photodynamic therapy. Biomaterials 2018, 182, 269–278.
- Ye, D.; Shuhendler, A.J.; Pandit, P.; Brewer, K.D.; Tee, S.S.; Cui, L.; Tikhomirov, G.; Rutt, B.; Rao, J. Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis. Chem. Sci. 2014, 5, 3845–3852.
- Yuan, Y.; Ding, Z.; Qian, J.; Zhang, J.; Xu, J.; Dong, X.; Han, T.; Ge, S.; Luo, Y.; Wang, Y.; et al. Casp3/7-instructed intracellular aggregation of Fe3O4 nanoparticles enhances T2 MR imaging of tumor apoptosis. Nano Lett. 2016, 16, 2686–2691.
- Ding, Z.; Sun, H.; Ge, S.; Cai, Y.; Yuan, Y.; Hai, Z.; Tao, T.; Hu, J.; Hu, B.; Wang, J.; et al. Furin-controlled Fe3O4 nanoparticle aggregation and 19F signal “turn-on” for precise MR imaging of tumors. Adv. Funct. Mater. 2019, 29, 1903860.
- Zheng, Z.; Sun, H.; Hu, C.; Li, G.; Liu, X.; Chen, P.; Cui, Y.; Liu, J.; Wang, J.; Liang, G. Using “ON/OFF” 19F NMR/magnetic resonance imaging signals to sense tyrosine kinase/phosphatase activity in vitro and in cell lysates. Anal. Chem. 2016, 88, 3363–3368.
- Liu, Y.; Miao, Q.; Zou, P.; Liu, L.; Wang, X.; An, L.; Zhang, X.; Qian, X.; Luo, S.; Liang, G. Enzyme-controlled intracellular self-assembly of 18F nanoparticles for enhanced microPET imaging of tumor. Theranostics 2015, 5, 1058–1067.
- Wang, H.; Chen, P.; Wu, H.; Zou, P.; Wu, J.; Liu, Y.; Liang, G. Furin-guided intracellular 68Ga nanoparticle formation enhancing tumor microPET imaging. Anal. Chem. 2019, 91, 14842–14845.
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524.
- Sun, I.C.; Eun, D.K.; Koo, H.; Ko, C.Y.; Kim, H.S.; Yi, D.K.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. Tumor-targeting gold particles for dual computed tomography/optical cancer imaging. Angew. Chem. Int. Ed. 2011, 50, 9348–9351.
- Bellat, V.; Ting, R.; Southard, T.L.; Vahdat, L.; Molina, H.; Fernandez, J.; Aras, O.; Stokol, T.; Law, B. Functional peptide nanofibers with unique tumor targeting and enzyme-induced local retention properties. Adv. Funct. Mater. 2018, 28, 1803969.
- Mao, W.; Kim, H.S.; Son, Y.J.; Kim, S.R.; Yoo, H.S. Doxorubicin encapsulated clicked gold nanoparticle clusters exhibiting tumor-specific disassembly for enhanced tumor localization and computerized tomographic imaging. J. Control. Release 2018, 269, 52–62.
- Yan, R.; Hu, Y.; Liu, F.; Wei, S.; Fang, D.; Shuhendler, A.J.; Liu, H.; Chen, H.Y.; Ye, D. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 2019, 141, 10331–10341.
This entry is offline, you can click here to edit this entry!
