Arthropods (/ˈɑːrθrəpɒd/, from grc ἄρθρον (arthron) 'joint', and πούς (pous) 'foot' (gen. ποδός)) are invertebrate animals having an exoskeleton, a segmented body, and paired jointed appendages. Arthropods form the phylum Arthropoda. They are distinguished by their jointed limbs and cuticle made of chitin, often mineralised with calcium carbonate. The arthropod body plan consists of segments, each with a pair of appendages. Arthropods are bilaterally symmetrical and their body possesses an external skeleton. In order to keep growing, they must go through stages of moulting, a process by which they shed their exoskeleton to reveal a new one. Some species have wings. They are an extremely diverse group, with up to 10 million species. The haemocoel, an arthropod's internal cavity, through which its haemolymph – analogue of blood – circulates, accommodates its interior organs; it has an open circulatory system. Like their exteriors, the internal organs of arthropods are generally built of repeated segments. Their nervous system is "ladder-like", with paired ventral nerve cords running through all segments and forming paired ganglia in each segment. Their heads are formed by fusion of varying numbers of segments, and their brains are formed by fusion of the ganglia of these segments and encircle the esophagus. The respiratory and excretory systems of arthropods vary, depending as much on their environment as on the subphylum to which they belong. Arthropods use combinations of compound eyes and pigment-pit ocelli for vision. In most species, the ocelli can only detect the direction from which light is coming, and the compound eyes are the main source of information, but the main eyes of spiders are ocelli that can form images and, in a few cases, can swivel to track prey. Arthropods also have a wide range of chemical and mechanical sensors, mostly based on modifications of the many bristles known as setae that project through their cuticles. Similarly, their reproduction and development are varied; all terrestrial species use internal fertilization, but this is sometimes by indirect transfer of the sperm via an appendage or the ground, rather than by direct injection. Aquatic species use either internal or external fertilization. Almost all arthropods lay eggs, but many species give birth to live young after the eggs have hatched inside the mother, and a few are genuinely viviparous, such as aphids. Arthropod hatchlings vary from miniature adults to grubs and caterpillars that lack jointed limbs and eventually undergo a total metamorphosis to produce the adult form. The level of maternal care for hatchlings varies from nonexistent to the prolonged care provided by social insects. The evolutionary ancestry of arthropods dates back to the Cambrian period. The group is generally regarded as monophyletic, and many analyses support the placement of arthropods with cycloneuralians (or their constituent clades) in a superphylum Ecdysozoa. Overall, however, the basal relationships of animals are not yet well resolved. Likewise, the relationships between various arthropod groups are still actively debated. Today, Arthropods contribute to the human food supply both directly as food, and more importantly, indirectly as pollinators of crops. Some species are known to spread severe disease to humans, livestock, and crops.
- ganglia
- ocelli
- superphylum
1. Etymology
The word arthropod comes from the Greek ἄρθρον árthron, "joint", and πούς pous (gen. podos (ποδός)), i.e. "foot" or "leg", which together mean "jointed leg".[1] The designation "Arthropoda" was coined in 1848 by the German physiologist and zoologist Karl Theodor Ernst von Siebold (1804–1885).[2][3]
In common parlance, terrestrial arthropods are often called bugs.[4] The term is also occasionally extended to colloquial names for freshwater or marine crustaceans (e.g. Balmain bug, Moreton Bay bug, mudbug) and used by physicians and bacteriologists for disease-causing germs (e.g. superbugs),[5] but entomologists reserve this term for a narrow category of "true bugs", insects of the order Hemiptera[5] (which does not include ants, bees, beetles, butterflies or moths).
2. Description
Arthropods are invertebrates with segmented bodies and jointed limbs.[6] The exoskeleton or cuticles consists of chitin, a polymer of glucosamine.[7] The cuticle of many crustaceans, beetle mites, and millipedes (except for bristly millipedes) is also biomineralized with calcium carbonate. Calcification of the endosternite, an internal structure used for muscle attachments, also occur in some opiliones.[8]
2.1. Diversity
Estimates of the number of arthropod species vary between 1,170,000 and 5 to 10 million and account for over 80 percent of all known living animal species.[9][10] The number of species remains difficult to determine. This is due to the census modeling assumptions projected onto other regions in order to scale up from counts at specific locations applied to the whole world. A study in 1992 estimated that there were 500,000 species of animals and plants in Costa Rica alone, of which 365,000 were arthropods.[11]
They are important members of marine, freshwater, land and air ecosystems, and are one of only two major animal groups that have adapted to life in dry environments; the other is amniotes, whose living members are reptiles, birds and mammals.[12] One arthropod sub-group, insects, is the most species-rich member of all ecological guilds in land and freshwater environments.[11] The lightest insects weigh less than 25 micrograms (millionths of a gram),[13] while the heaviest weigh over 70 grams (2 1⁄2 oz).[14] Some living malacostracans are much larger; for example, the legs of the Japanese spider crab may span up to 4 metres (13 ft),[13] with the heaviest of all living arthropods being the American lobster, topping out at over 20 kg (44 lbs).
2.2. Segmentation
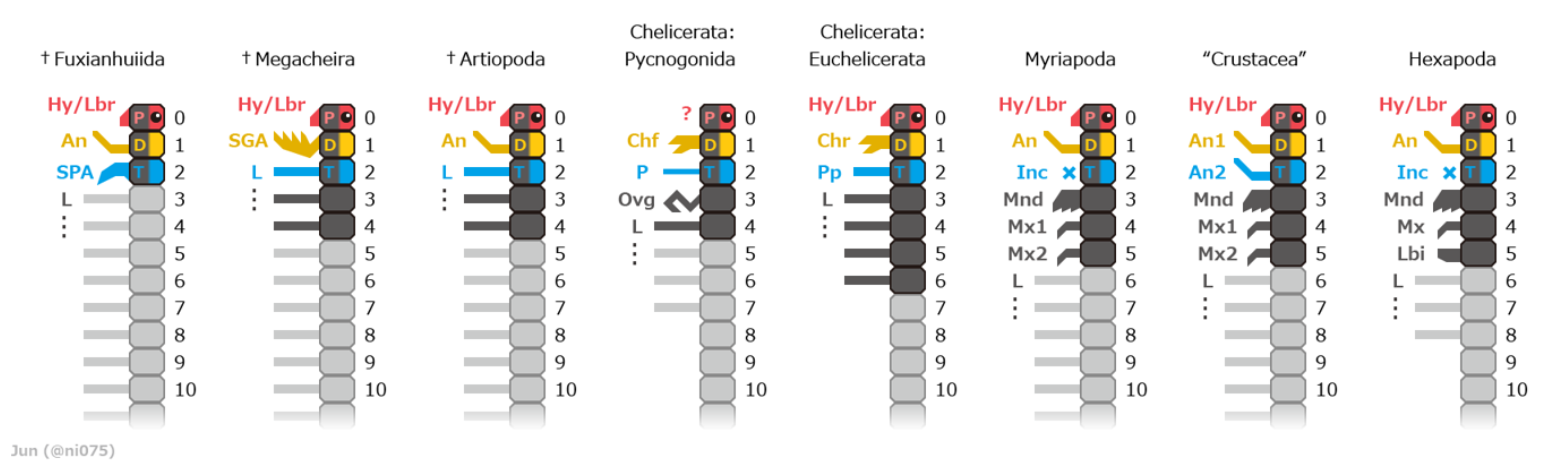
The embryos of all arthropods are segmented, built from a series of repeated modules. The last common ancestor of living arthropods probably consisted of a series of undifferentiated segments, each with a pair of appendages that functioned as limbs. However, all known living and fossil arthropods have grouped segments into tagmata in which segments and their limbs are specialized in various ways.[12]
The three-part appearance of many insect bodies and the two-part appearance of spiders is a result of this grouping;[16] in fact there are no external signs of segmentation in mites.[12] Arthropods also have two body elements that are not part of this serially repeated pattern of segments, an ocular somite at the front, where the mouth and eyes originated,[12][17] and a telson at the rear, behind the anus.
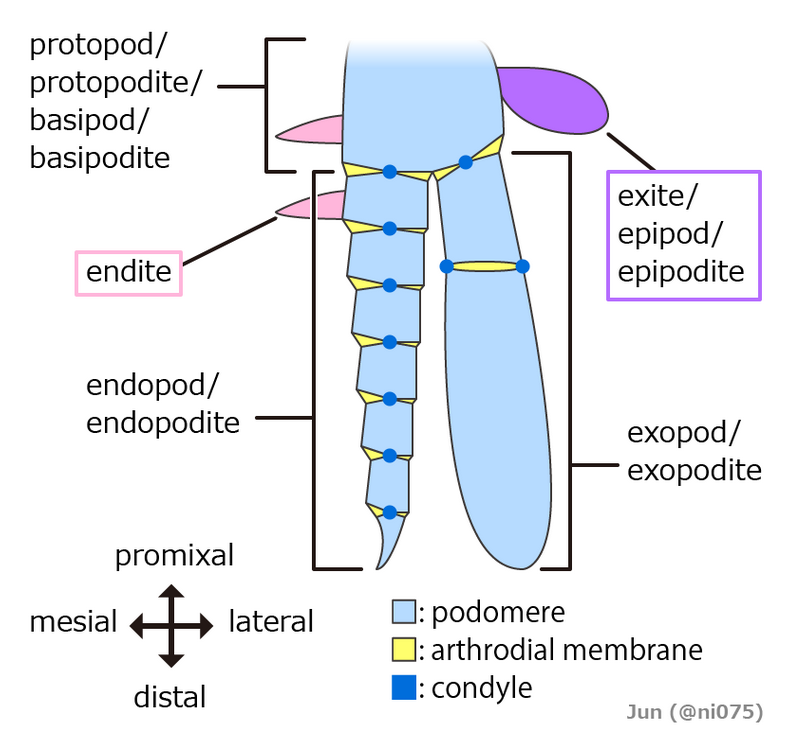
Originally it seems that each appendage-bearing segment had two separate pairs of appendages: an upper, unsegmented exite and a lower, segmented endopod. These would later fuse into a single pair of biramous appendages united by a basal segment (protopod or basipod), with the upper branch acting as a gill while the lower branch was used for locomotion.[15][18][19] The appendages of most crustaceans and some extinct taxa such as trilobites have another segmented branch known as exopods, but whether these structures have a single origin remain controversial.[15][20][21] In some segments of all known arthropods the appendages have been modified, for example to form gills, mouth-parts, antennae for collecting information,[16] or claws for grasping;[22] arthropods are "like Swiss Army knives, each equipped with a unique set of specialized tools."[12] In many arthropods, appendages have vanished from some regions of the body; it is particularly common for abdominal appendages to have disappeared or be highly modified.[12]
The most conspicuous specialization of segments is in the head. The four major groups of arthropods – Chelicerata (sea spiders, horseshoe crabs and arachnids), Myriapoda (symphylan, pauropods, millipedes and centipedes), Crustacea (oligostracans, copepods, malacostracans, branchiopods, hexapods, etc.), and the extinct Trilobita – have heads formed of various combinations of segments, with appendages that are missing or specialized in different ways.[12] Despite myriapods and hexapods both having similar head combinations, hexapods are deeply nested within crustacea while myriapods are not, so these traits are believed to have evolved separately. In addition, some extinct arthropods, such as Marrella, belong to none of these groups, as their heads are formed by their own particular combinations of segments and specialized appendages.[24]
Working out the evolutionary stages by which all these different combinations could have appeared is so difficult that it has long been known as "the arthropod head problem".[25] In 1960, R. E. Snodgrass even hoped it would not be solved, as he found trying to work out solutions to be fun.[26]
2.3. Exoskeleton
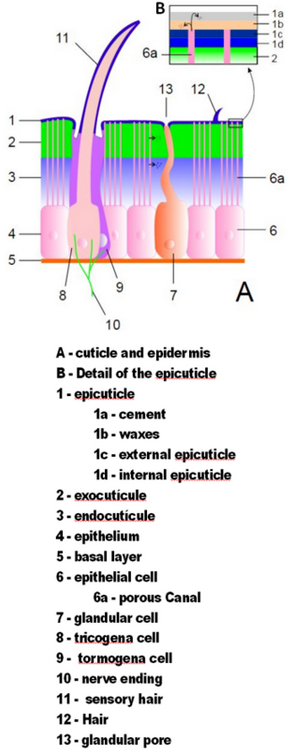
Arthropod exoskeletons are made of cuticle, a non-cellular material secreted by the epidermis.[12] Their cuticles vary in the details of their structure, but generally consist of three main layers: the epicuticle, a thin outer waxy coat that moisture-proofs the other layers and gives them some protection; the exocuticle, which consists of chitin and chemically hardened proteins; and the endocuticle, which consists of chitin and unhardened proteins. The exocuticle and endocuticle together are known as the procuticle.[27] Each body segment and limb section is encased in hardened cuticle. The joints between body segments and between limb sections are covered by flexible cuticle.[12]
The exoskeletons of most aquatic crustaceans are biomineralized with calcium carbonate extracted from the water. Some terrestrial crustaceans have developed means of storing the mineral, since on land they cannot rely on a steady supply of dissolved calcium carbonate.[28] Biomineralization generally affects the exocuticle and the outer part of the endocuticle.[27] Two recent hypotheses about the evolution of biomineralization in arthropods and other groups of animals propose that it provides tougher defensive armor,[29] and that it allows animals to grow larger and stronger by providing more rigid skeletons;[30] and in either case a mineral-organic composite exoskeleton is cheaper to build than an all-organic one of comparable strength.[30][31]
The cuticle may have setae (bristles) growing from special cells in the epidermis. Setae are as varied in form and function as appendages. For example, they are often used as sensors to detect air or water currents, or contact with objects; aquatic arthropods use feather-like setae to increase the surface area of swimming appendages and to filter food particles out of water; aquatic insects, which are air-breathers, use thick felt-like coats of setae to trap air, extending the time they can spend under water; heavy, rigid setae serve as defensive spines.[12]
Although all arthropods use muscles attached to the inside of the exoskeleton to flex their limbs, some still use hydraulic pressure to extend them, a system inherited from their pre-arthropod ancestors;[32] for example, all spiders extend their legs hydraulically and can generate pressures up to eight times their resting level.[33]
2.4. Moulting

The exoskeleton cannot stretch and thus restricts growth. Arthropods, therefore, replace their exoskeletons by undergoing ecdysis (moulting), or shedding the old exoskeleton after growing a new one that is not yet hardened. Moulting cycles run nearly continuously until an arthropod reaches full size.[34]
The developmental stages between each moult (ecdysis) until sexual maturity is reached is called an instar. Differences between instars can often be seen in altered body proportions, colors, patterns, changes in the number of body segments or head width. After moulting, i.e. shedding their exoskeleton, the juvenile arthropods continue in their life cycle until they either pupate or moult again.
In the initial phase of moulting, the animal stops feeding and its epidermis releases moulting fluid, a mixture of enzymes that digests the endocuticle and thus detaches the old cuticle. This phase begins when the epidermis has secreted a new epicuticle to protect it from the enzymes, and the epidermis secretes the new exocuticle while the old cuticle is detaching. When this stage is complete, the animal makes its body swell by taking in a large quantity of water or air, and this makes the old cuticle split along predefined weaknesses where the old exocuticle was thinnest. It commonly takes several minutes for the animal to struggle out of the old cuticle. At this point, the new one is wrinkled and so soft that the animal cannot support itself and finds it very difficult to move, and the new endocuticle has not yet formed. The animal continues to pump itself up to stretch the new cuticle as much as possible, then hardens the new exocuticle and eliminates the excess air or water. By the end of this phase, the new endocuticle has formed. Many arthropods then eat the discarded cuticle to reclaim its materials.[34]
Because arthropods are unprotected and nearly immobilized until the new cuticle has hardened, they are in danger both of being trapped in the old cuticle and of being attacked by predators. Moulting may be responsible for 80 to 90% of all arthropod deaths.[34]
2.5. Internal Organs

Arthropod bodies are also segmented internally, and the nervous, muscular, circulatory, and excretory systems have repeated components.[12] Arthropods come from a lineage of animals that have a coelom, a membrane-lined cavity between the gut and the body wall that accommodates the internal organs. The strong, segmented limbs of arthropods eliminate the need for one of the coelom's main ancestral functions, as a hydrostatic skeleton, which muscles compress in order to change the animal's shape and thus enable it to move. Hence the coelom of the arthropod is reduced to small areas around the reproductive and excretory systems. Its place is largely taken by a hemocoel, a cavity that runs most of the length of the body and through which blood flows.[35]
2.6. Respiration and Circulation
Arthropods have open circulatory systems, although most have a few short, open-ended arteries. In chelicerates and crustaceans, the blood carries oxygen to the tissues, while hexapods use a separate system of tracheae. Many crustaceans, but few chelicerates and tracheates, use respiratory pigments to assist oxygen transport. The most common respiratory pigment in arthropods is copper-based hemocyanin; this is used by many crustaceans and a few centipedes. A few crustaceans and insects use iron-based hemoglobin, the respiratory pigment used by vertebrates. As with other invertebrates, the respiratory pigments of those arthropods that have them are generally dissolved in the blood and rarely enclosed in corpuscles as they are in vertebrates.[35]
The heart is typically a muscular tube that runs just under the back and for most of the length of the hemocoel. It contracts in ripples that run from rear to front, pushing blood forwards. Sections not being squeezed by the heart muscle are expanded either by elastic ligaments or by small muscles, in either case connecting the heart to the body wall. Along the heart run a series of paired ostia, non-return valves that allow blood to enter the heart but prevent it from leaving before it reaches the front.[35]
Arthropods have a wide variety of respiratory systems. Small species often do not have any, since their high ratio of surface area to volume enables simple diffusion through the body surface to supply enough oxygen. Crustacea usually have gills that are modified appendages. Many arachnids have book lungs.[36] Tracheae, systems of branching tunnels that run from the openings in the body walls, deliver oxygen directly to individual cells in many insects, myriapods and arachnids.[37]
2.7. Nervous System
Living arthropods have paired main nerve cords running along their bodies below the gut, and in each segment the cords form a pair of ganglia from which sensory and motor nerves run to other parts of the segment. Although the pairs of ganglia in each segment often appear physically fused, they are connected by commissures (relatively large bundles of nerves), which give arthropod nervous systems a characteristic "ladder-like" appearance. The brain is in the head, encircling and mainly above the esophagus. It consists of the fused ganglia of the acron and one or two of the foremost segments that form the head – a total of three pairs of ganglia in most arthropods, but only two in chelicerates, which do not have antennae or the ganglion connected to them. The ganglia of other head segments are often close to the brain and function as part of it. In insects these other head ganglia combine into a pair of subesophageal ganglia, under and behind the esophagus. Spiders take this process a step further, as all the segmental ganglia are incorporated into the subesophageal ganglia, which occupy most of the space in the cephalothorax (front "super-segment").[38]
2.8. Excretory System
There are two different types of arthropod excretory systems. In aquatic arthropods, the end-product of biochemical reactions that metabolise nitrogen is ammonia, which is so toxic that it needs to be diluted as much as possible with water. The ammonia is then eliminated via any permeable membrane, mainly through the gills.[36] All crustaceans use this system, and its high consumption of water may be responsible for the relative lack of success of crustaceans as land animals.[39] Various groups of terrestrial arthropods have independently developed a different system: the end-product of nitrogen metabolism is uric acid, which can be excreted as dry material; the Malpighian tubule system filters the uric acid and other nitrogenous waste out of the blood in the hemocoel, and dumps these materials into the hindgut, from which they are expelled as feces.[39] Most aquatic arthropods and some terrestrial ones also have organs called nephridia ("little kidneys"), which extract other wastes for excretion as urine.[39]
2.9. Senses
The stiff cuticles of arthropods would block out information about the outside world, except that they are penetrated by many sensors or connections from sensors to the nervous system. In fact, arthropods have modified their cuticles into elaborate arrays of sensors. Various touch sensors, mostly setae, respond to different levels of force, from strong contact to very weak air currents. Chemical sensors provide equivalents of taste and smell, often by means of setae. Pressure sensors often take the form of membranes that function as eardrums, but are connected directly to nerves rather than to auditory ossicles. The antennae of most hexapods include sensor packages that monitor humidity, moisture and temperature.[40]
Most arthropods lack balance and acceleration sensors, and rely on their eyes to tell them which way is up. The self-righting behavior of cockroaches is triggered when pressure sensors on the underside of the feet report no pressure. However, many malacostracan crustaceans have statocysts, which provide the same sort of information as the balance and motion sensors of the vertebrate inner ear.[40]
The proprioceptors of arthropods, sensors that report the force exerted by muscles and the degree of bending in the body and joints, are well understood. However, little is known about what other internal sensors arthropods may have.[40]
Optical


Most arthropods have sophisticated visual systems that include one or more usually both of compound eyes and pigment-cup ocelli ("little eyes"). In most cases ocelli are only capable of detecting the direction from which light is coming, using the shadow cast by the walls of the cup. However, the main eyes of spiders are pigment-cup ocelli that are capable of forming images,[40] and those of jumping spiders can rotate to track prey.[41]
Compound eyes consist of fifteen to several thousand independent ommatidia, columns that are usually hexagonal in cross section. Each ommatidium is an independent sensor, with its own light-sensitive cells and often with its own lens and cornea.[40] Compound eyes have a wide field of view, and can detect fast movement and, in some cases, the polarization of light.[42] On the other hand, the relatively large size of ommatidia makes the images rather coarse, and compound eyes are shorter-sighted than those of birds and mammals – although this is not a severe disadvantage, as objects and events within 20 cm (8 in) are most important to most arthropods.[40] Several arthropods have color vision, and that of some insects has been studied in detail; for example, the ommatidia of bees contain receptors for both green and ultra-violet.[40]
Olfaction
3. Reproduction and Development

A few arthropods, such as barnacles, are hermaphroditic, that is, each can have the organs of both sexes. However, individuals of most species remain of one sex their entire lives.[43] A few species of insects and crustaceans can reproduce by parthenogenesis, especially if conditions favor a "population explosion". However, most arthropods rely on sexual reproduction, and parthenogenetic species often revert to sexual reproduction when conditions become less favorable.[44] The ability to undergo meiosis is widespread among arthropods including both those that reproduce sexually and those that reproduce parthenogenetically.[45] Although meiosis is a major characteristic of arthropods, understanding of its fundamental adaptive benefit has long been regarded as an unresolved problem,[46] that appears to have remained unsettled.
Aquatic arthropods may breed by external fertilization, as for example frogs do, or by internal fertilization, where the ova remain in the female's body and the sperm must somehow be inserted. All known terrestrial arthropods use internal fertilization. Opiliones (harvestmen), millipedes, and some crustaceans use modified appendages such as gonopods or penises to transfer the sperm directly to the female. However, most male terrestrial arthropods produce spermatophores, waterproof packets of sperm, which the females take into their bodies. A few such species rely on females to find spermatophores that have already been deposited on the ground, but in most cases males only deposit spermatophores when complex courtship rituals look likely to be successful.[43]

Most arthropods lay eggs,[43] but scorpions are ovoviparous: they produce live young after the eggs have hatched inside the mother, and are noted for prolonged maternal care.[47] Newly born arthropods have diverse forms, and insects alone cover the range of extremes. Some hatch as apparently miniature adults (direct development), and in some cases, such as silverfish, the hatchlings do not feed and may be helpless until after their first moult. Many insects hatch as grubs or caterpillars, which do not have segmented limbs or hardened cuticles, and metamorphose into adult forms by entering an inactive phase in which the larval tissues are broken down and re-used to build the adult body.[48] Dragonfly larvae have the typical cuticles and jointed limbs of arthropods but are flightless water-breathers with extendable jaws.[49] Crustaceans commonly hatch as tiny nauplius larvae that have only three segments and pairs of appendages.[43]
4. Evolutionary History
4.1. Last Common Ancestor
Based on the distribution of shared plesiomorphic features in extant and fossil taxa, the last common ancestor of all arthropods is inferred to have been as a modular organism with each module covered by its own sclerite (armor plate) and bearing a pair of biramous limbs.[50] However, whether the ancestral limb was uniramous or biramous is far from a settled debate. This Ur-arthropod had a ventral mouth, pre-oral antennae and dorsal eyes at the front of the body. It was assumed to have been a non-discriminatory sediment feeder, processing whatever sediment came its way for food,[50] but fossil findings hint that the last common ancestor of both arthropods and priapulida shared the same specialized mouth apparatus; a circular mouth with rings of teeth used for capturing animal prey.[51]
4.2. Fossil Record

It has been proposed that the Ediacaran animals Parvancorina and Spriggina, from around 555 million years ago, were arthropods,[52][53][54] but later study shows that their affinities of being origin of arthropods are not reliable.[55] Small arthropods with bivalve-like shells have been found in Early Cambrian fossil beds dating 541 to 539 million years ago in China and Australia.[56][57][58][59] The earliest Cambrian trilobite fossils are about 530 million years old, but the class was already quite diverse and worldwide, suggesting that they had been around for quite some time.[60] In the Maotianshan shales, which date to between 530 and 520 million years ago, fossils of arthropods such as Kylinxia and Erratus have been found that seem to show a transitional split between lobopodia and other more primitive stem arthropods.[61][62] Re-examination in the 1970s of the Burgess Shale fossils from about 505 million years ago identified many arthropods, some of which could not be assigned to any of the well-known groups, and thus intensified the debate about the Cambrian explosion.[63][64][65] A fossil of Marrella from the Burgess Shale has provided the earliest clear evidence of moulting.[66]

The earliest fossil crustaceans date from about 511 million years ago in the Cambrian,[67] and fossil shrimp from about 500 million years ago apparently formed a tight-knit procession across the seabed.[68] Crustacean fossils are common from the Ordovician period onwards.Cite error: Closing </ref> missing for <ref> tag and terrestrial tracks from about 450 million years ago appear to have been made by arthropods.[69] Arthropods possessed attributes that were easy coopted for life on land; their existing jointed exoskeletons provided protection against desiccation, support against gravity and a means of locomotion that was not dependent on water.[70] Around the same time the aquatic, scorpion-like eurypterids became the largest ever arthropods, some as long as 2.5 m (8 ft 2 in).[71]
The oldest known arachnid is the trigonotarbid Palaeotarbus jerami, from about 420 million years ago in the Silurian period.[72][73] Attercopus fimbriunguis, from 386 million years ago in the Devonian period, bears the earliest known silk-producing spigots, but its lack of spinnerets means it was not one of the true spiders,[74] which first appear in the Late Carboniferous over 299 million years ago.[75] The Jurassic and Cretaceous periods provide a large number of fossil spiders, including representatives of many modern families.[76] Fossils of aquatic scorpions with gills appear in the Silurian and Devonian periods, and the earliest fossil of an air-breathing scorpion with book lungs dates from the Early Carboniferous period.[77]
The oldest possible insect fossil is the Devonian Rhyniognatha hirsti, dated at 396 to 407 million years ago, but its mandibles are of a type found only in winged insects, which suggests that the earliest insects appeared in the Silurian period,[78] although later study shows possibility that Rhyniognatha can be myriapod, not an insect.[79] The Mazon Creek lagerstätten from the Late Carboniferous, about 300 million years ago, include about 200 species, some gigantic by modern standards, and indicate that insects had occupied their main modern ecological niches as herbivores, detritivores and insectivores. Social termites and ants first appear in the Early Cretaceous, and advanced social bees have been found in Late Cretaceous rocks but did not become abundant until the Middle Cenozoic.[80]
4.3. Evolutionary Family Tree

From 1952 to 1977, zoologist Sidnie Manton and others argued that arthropods are polyphyletic, in other words, that they do not share a common ancestor that was itself an arthropod. Instead, they proposed that three separate groups of "arthropods" evolved separately from common worm-like ancestors: the chelicerates, including spiders and scorpions; the crustaceans; and the uniramia, consisting of onychophorans, myriapods and hexapods. These arguments usually bypassed trilobites, as the evolutionary relationships of this class were unclear. Proponents of polyphyly argued the following: that the similarities between these groups are the results of convergent evolution, as natural consequences of having rigid, segmented exoskeletons; that the three groups use different chemical means of hardening the cuticle; that there were significant differences in the construction of their compound eyes; that it is hard to see how such different configurations of segments and appendages in the head could have evolved from the same ancestor; and that crustaceans have biramous limbs with separate gill and leg branches, while the other two groups have uniramous limbs in which the single branch serves as a leg.[82]
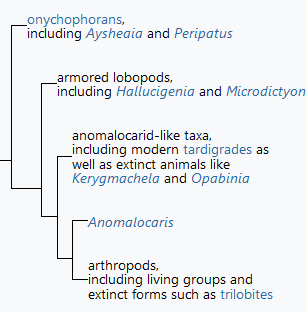
Simplified summary of Budd's "broad-scale" cladogram (1996)[81]
Further analysis and discoveries in the 1990s reversed this view, and led to acceptance that arthropods are monophyletic, in other words they are inferred to share a common ancestor that was itself an arthropod.[83][84] For example, Graham Budd's analyses of Kerygmachela in 1993 and of Opabinia in 1996 convinced him that these animals were similar to onychophorans and to various Early Cambrian "lobopods", and he presented an "evolutionary family tree" that showed these as "aunts" and "cousins" of all arthropods.[81][85] These changes made the scope of the term "arthropod" unclear, and Claus Nielsen proposed that the wider group should be labelled "Panarthropoda" ("all the arthropods") while the animals with jointed limbs and hardened cuticles should be called "Euarthropoda" ("true arthropods").[86]
A contrary view was presented in 2003, when Jan Bergström and Xian-Guang Hou argued that, if arthropods were a "sister-group" to any of the anomalocarids, they must have lost and then re-evolved features that were well-developed in the anomalocarids. The earliest known arthropods ate mud in order to extract food particles from it, and possessed variable numbers of segments with unspecialized appendages that functioned as both gills and legs. Anomalocarids were, by the standards of the time, huge and sophisticated predators with specialized mouths and grasping appendages, fixed numbers of segments some of which were specialized, tail fins, and gills that were very different from those of arthropods. In 2006, they suggested that arthropods were more closely related to lobopods and tardigrades than to anomalocarids.[87] In 2014, research indicated that tardigrades were more closely related to arthropods than velvet worms.[88]
Higher up the "family tree", the Annelida have traditionally been considered the closest relatives of the Panarthropoda, since both groups have segmented bodies, and the combination of these groups was labelled Articulata. There had been competing proposals that arthropods were closely related to other groups such as nematodes, priapulids and tardigrades, but these remained minority views because it was difficult to specify in detail the relationships between these groups.
In the 1990s, molecular phylogenetic analyses of DNA sequences produced a coherent scheme showing arthropods as members of a superphylum labelled Ecdysozoa ("animals that moult"), which contained nematodes, priapulids and tardigrades but excluded annelids. This was backed up by studies of the anatomy and development of these animals, which showed that many of the features that supported the Articulata hypothesis showed significant differences between annelids and the earliest Panarthropods in their details, and some were hardly present at all in arthropods. This hypothesis groups annelids with molluscs and brachiopods in another superphylum, Lophotrochozoa.
If the Ecdysozoa hypothesis is correct, then segmentation of arthropods and annelids either has evolved convergently or has been inherited from a much older ancestor and subsequently lost in several other lineages, such as the non-arthropod members of the Ecdysozoa.[89][91]
4.4. Phylogeny of Stem-Group Arthropods
Modern interpretations of the basal, extinct stem-group of Arthropoda recognised the following groups, from most basal to most crownward:[92][93]
- The "Giant" or "Siberiid Lobopodians", such as Jianshanopodia, Siberion and Megadictyon, are the most basal grade in the total-group Arthropoda.
- The "Gilled Lobopodians", such as Kerygmachela, Pambdelurion and Opabinia, are the second most basal grade.
- The Radiodonta, which traditionally known as anomalocaridids come in third position, and are thought to be monophyletic.
- A possible "upper stem-group" assemblage of more uncertain position[93] but contained within Deuteropoda:[92] the Fuxianhuiida, Megacheira, and multiple "bivalved forms" including Isoxyida (Isoxys and Surusicaris) and Hymenocarina.
The Deuteropoda is a recently established clade uniting the crown-group (living) arthropods with these possible "upper stem-group" fossils taxa.[92] The clade is defined by important changes to the structure of the head region such as the appearance of a differentiated deutocerebral appendage pair.[92]
However, recent analyses since late 2010s also show that these "upper stem-groups" might be inside the crown-group:[93] isoxyids might nested with the crown-group itself,[94][95] Megacheira have been recovered as more closely related to Chelicerates,[94][95] some bivalved forms such as Hymenocarina are consistently shown to be mandibulates,[93] and similarly Fuxianhuiida might also be mandibulates as well.[96]
The following cladogram shows the probable relationships between crown-group Arthropoda and stem-group Arthropoda according to O’Flynn et al. 2022, including two new fossils found to be the most early branches of Deuteropoda[94][95] (the "upper stem-groups" in previous studies[92] are marked in asterisk, living groups are marked in bold):
Note that the subphylum Artiopoda, containing the trilobites, is closer to mandibulates than to chelicerates in the cladogram above,[94][95] but older analyses place them as the sister group of chelicerates[93] united under the clade Arachnomorpha.
4.5. Phylogeny of Living Arthropods
The following cladogram shows the internal relationships between all the living classes of arthropods as of late 2010s,[97][98] as well as the estimated timing for some of the clades:[99]
5. Classification
The phylum Arthropoda is typically subdivided into four subphyla, of which one is extinct:[100]
- Artiopods were an extinct group of formerly numerous marine animals that disappeared in the Permian–Triassic extinction event, though they were in decline prior to this killing blow, having been reduced to one order in the Late Devonian extinction. They contain groups such as the trilobites.
- Chelicerates comprise the marine sea spiders and horseshoe crabs, along with the terrestrial arachnids such as mites, harvestmen, spiders, scorpions and related organisms characterized by the presence of chelicerae, appendages just above/in front of the mouthparts. Chelicerae appear in scorpions and horseshoe crabs as tiny claws that they use in feeding, but those of spiders have developed as fangs that inject venom.
- Myriapods comprise millipedes, centipedes, pauropods and symphylans, characterized by having numerous body segments each of which bearing one or two pairs of legs (or in a few cases being legless). All members are exclusively terrestrial.
- Pancrustaceans comprise ostracods, barnacles, copepods, malacostracans, cephalocaridans, branchiopods, remipedes and hexapods. Most groups are primarily aquatic (two notable exceptions being woodlice and hexapods, which are both purely terrestrial) and are characterized by having biramous appendages. The most abundant group of pancrustaceans are the terrestrial hexapods, which comprise insects, diplurans, springtails, and proturans, with six thoracic legs.
Aside from these major groups, a number of fossil forms, mostly from the early Cambrian period, are difficult to place taxonomically, either from lack of obvious affinity to any of the main groups or from clear affinity to several of them. Marrella was the first one to be recognized as significantly different from the well-known groups.[24]
The phylogeny of the major extant arthropod groups has been an area of considerable interest and dispute.[101] Recent studies strongly suggest that Crustacea, as traditionally defined, is paraphyletic, with Hexapoda having evolved from within it,[102][103] so that Crustacea and Hexapoda form a clade, Pancrustacea. The position of Myriapoda, Chelicerata and Pancrustacea remains unclear (As of April 2012). In some studies, Myriapoda is grouped with Chelicerata (forming Myriochelata);[104][105] in other studies, Myriapoda is grouped with Pancrustacea (forming Mandibulata),[102] or Myriapoda may be sister to Chelicerata plus Pancrustacea.[103]
The placement of the extinct trilobites is also a frequent subject of dispute.[106] One of the newer hypotheses is that the chelicerae have originated from the same pair of appendages that evolved into antennae in the ancestors of Mandibulata, which would place trilobites, which had antennae, closer to Mandibulata than Chelicerata.[107]
Since the International Code of Zoological Nomenclature recognises no priority above the rank of family, many of the higher-level groups can be referred to by a variety of different names.[108]
| Subphyla | Classes | Members | Example 1 |
|---|---|---|---|
| Chelicerata | Pycnogonida Xiphosura Arachnida |
Sea Spiders Horseshoe Crabs Harvestmen, Solifuges, Mites, Scorpions, Spiders, Ticks etc. |
 Platycryptus undatus (Arachnida, Araneae) |
| Myriapoda | Symphyla Pauropoda Diplopoda Chilopoda |
Pseudocentipedes Hexameroceratans, Tetrameroceratans Bristle Millipedes, Pill Millipedes, Flat-Backed Millipedes, etc. Scutigeromorphs, Lithobiomorphs, Scolopendromorphs, etc. |
 Archispirostreptus gigas (Diplopoda, Spirostreptida) |
| Crustacea | Ostracoda Mystacocarida Pentastomida Branchiura Thecostraca Copepoda Malacostraca Cephalocarida Branchiopoda Remipedia |
Seed Shrimp Mystacocaridans Tongue Worms Fish Lice Barnacles, etc. Calanoids, Cyclopoids, Misophrioids, Siphonostomatoids, etc. Mantis Shrimp, Skeleton Shrimp, Woodlice, Shrimp, Crabs, Krill, etc. Horseshoe Shrimp Fairy Shrimp, Tadpole Shrimp, Water Fleas, Clam Shrimp Remipedes |
 Ocypode ceratophthalma (Malacostraca, Decapoda) |
| Hexapoda | Insecta Entognatha |
Insects Springtails, etc. |
 Saturnia pavonia (Insecta, Lepidoptera) |
6. Interaction with Humans

Crustaceans such as crabs, lobsters, crayfish, shrimp, and prawns have long been part of human cuisine, and are now raised commercially.[109] Insects and their grubs are at least as nutritious as meat, and are eaten both raw and cooked in many cultures, though not most European, Hindu, and Islamic cultures.[110][111] Cooked tarantulas are considered a delicacy in Cambodia,[112][113][114] and by the Piaroa Indians of southern Venezuela, after the highly irritant hairs – the spider's main defense system – are removed.[115] Humans also unintentionally eat arthropods in other foods,[116] and food safety regulations lay down acceptable contamination levels for different kinds of food material.[117][118] The intentional cultivation of arthropods and other small animals for human food, referred to as minilivestock, is now emerging in animal husbandry as an ecologically sound concept.[119] Commercial butterfly breeding provides Lepidoptera stock to butterfly conservatories, educational exhibits, schools, research facilities, and cultural events.
However, the greatest contribution of arthropods to human food supply is by pollination: a 2008 study examined the 100 crops that FAO lists as grown for food, and estimated pollination's economic value as €153 billion, or 9.5 per cent of the value of world agricultural production used for human food in 2005.[120] Besides pollinating, bees produce honey, which is the basis of a rapidly growing industry and international trade.[121]
The red dye cochineal, produced from a Central American species of insect, was economically important to the Aztecs and Mayans.[122] While the region was under Spain control, it became Mexico's second most-lucrative export,[123] and is now regaining some of the ground it lost to synthetic competitors.[124] Shellac, a resin secreted by a species of insect native to southern Asia, was historically used in great quantities for many applications in which it has mostly been replaced by synthetic resins, but it is still used in woodworking and as a food additive. The blood of horseshoe crabs contains a clotting agent, Limulus Amebocyte Lysate, which is now used to test that antibiotics and kidney machines are free of dangerous bacteria, and to detect spinal meningitis and some cancers.[125] Forensic entomology uses evidence provided by arthropods to establish the time and sometimes the place of death of a human, and in some cases the cause.[126] Recently insects have also gained attention as potential sources of drugs and other medicinal substances.[127]
The relative simplicity of the arthropods' body plan, allowing them to move on a variety of surfaces both on land and in water, have made them useful as models for robotics. The redundancy provided by segments allows arthropods and biomimetic robots to move normally even with damaged or lost appendages.[128][129]
| Disease[130] | Insect | Cases per year | Deaths per year |
|---|---|---|---|
| Malaria | Anopheles mosquito | 267 M | 1 to 2 M |
| Dengue fever | Aedes mosquito | ? | ? |
| Yellow fever | Aedes mosquito | 4,432 | 1,177 |
| Filariasis | Culex mosquito | 250 M | unknown |
Although arthropods are the most numerous phylum on Earth, and thousands of arthropod species are venomous, they inflict relatively few serious bites and stings on humans. Far more serious are the effects on humans of diseases like malaria carried by blood-sucking insects. Other blood-sucking insects infect livestock with diseases that kill many animals and greatly reduce the usefulness of others.[130] Ticks can cause tick paralysis and several parasite-borne diseases in humans.[131] A few of the closely related mites also infest humans, causing intense itching,[132] and others cause allergic diseases, including hay fever, asthma, and eczema.[133]
Many species of arthropods, principally insects but also mites, are agricultural and forest pests.[134][135] The mite Varroa destructor has become the largest single problem faced by beekeepers worldwide.[136] Efforts to control arthropod pests by large-scale use of pesticides have caused long-term effects on human health and on biodiversity.[137] Increasing arthropod resistance to pesticides has led to the development of integrated pest management using a wide range of measures including biological control.[134] Predatory mites may be useful in controlling some mite pests.[138][139]
7. As Predators
Even amongst arthropods usually thought of as obligate predators, floral food sources (nectar and to a lesser degree pollen) are often useful adjunct sources.[140] It was noticed in one study[141] that adult Adalia bipunctata (predator and common biocontrol of Ephestia kuehniella) could survive on flowers but never completed the life cycle, so a meta-analysis[140] was done to find such an overall trend in previously published data, if it existed. In some cases floral resources are outright necessary.[140] Overall, floral resources (and an imitation, i.e. sugar water) increase longevity and fecundity, meaning even predatory population numbers can depend on non-prey food abundance.[140] Thus biocontrol success may surprisingly depend on nearby flowers.[140]
The content is sourced from: https://handwiki.org/wiki/Biology:Arthropod
References
- "Arthropoda". Online Etymology Dictionary. http://www.etymonline.com/index.php?term=Arthropoda&allowed_in_frame=0.
- Siebold, C. Th. v. (1848) (in de). Lehrbuch der vergleichenden Anatomie der Wirbellosen Thiere. Berlin, (Germany): Veit & Co.. p. 4. https://archive.org/details/lehrbuchdervergl01sieb. From p. 4: "Arthropoda. Thiere mit vollkommen symmetrischer Form und gegliederten Bewegungsorganen. Centralmasse des Nervensystems besteht aus einem den Schlund umfassenden Ganglienring und einer von diesem ausgehenden Bauch-Ganglienkette." (Arthropoda. Animals with completely symmetric form and articulated organs of movement. Central mass of the nervous system consists of a ring of ganglia surrounding the esophagus and an abdominal chain of ganglia extending from this [ring of ganglia].)
- Hegna, Thomas A.; Legg, David A.; Møller, Ole Sten; Van Roy, Peter; Lerosey-Aubril, Rudy (November 19, 2013). "The correct authorship of the taxon name 'Arthropoda'". Arthropod Systematics & Phylogeny 71 (2): 71–74.
- The Museum of New Zealand notes that "in everyday conversation", bug "refers to land arthropods with at least six legs, such as insects, spiders, and centipedes".[8] In a chapter on "Bugs That Are Not Insects", entomologist Gilbert Walbauer specifies centipedes, millipedes, arachnids (spiders, daddy longlegs, scorpions, mites, chiggers and ticks) as well as the few terrestrial crustaceans (sowbugs and pillbugs),[9] but argues that "including legless creatures such as worms, slugs, and snails among the bugs stretches the word too much".[10]
- Gilbert Waldbauer. The Handy Bug Answer Book. Visible Ink, 1998. p. 1. ISBN:9781578590490 https://archive.org/details/handybuganswerbo00wald/page/1/mode/2up
- Valentine, J. W. (2004), On the Origin of Phyla, University of Chicago Press, p. 33, ISBN 978-0-226-84548-7, https://books.google.com/books?id=DMBkmHm5fe4C&q=arthropod+synapomorphy
- Cutler, B. (August 1980), "Arthropod cuticle features and arthropod monophyly", Cellular and Molecular Life Sciences 36 (8): 953, doi:10.1007/BF01953812 https://dx.doi.org/10.1007%2FBF01953812
- Kovoor, J. (1978). "Natural calcification of the prosomatic endosternite in the Phalangiidae (Arachnida:Opiliones).". Calcified Tissue Research 26 (3): 267–9. doi:10.1007/BF02013269. PMID 750069. https://dx.doi.org/10.1007%2FBF02013269
- Thanukos, Anna, The Arthropod Story, University of California, Berkeley, http://evolution.berkeley.edu/evolibrary/article/arthropodstory, retrieved 2008-09-29
- Ødegaard, Frode (December 2000), "How many species of arthropods? Erwin's estimate revised.", Biological Journal of the Linnean Society 71 (4): 583–597, doi:10.1006/bijl.2000.0468, http://si-pddr.si.edu/dspace/bitstream/10088/1315/1/Odegaard_2000.pdf, retrieved 2010-05-06
- Thompson, J. N. (1994), The Coevolutionary Process, University of Chicago Press, p. 9, ISBN 978-0-226-79760-1, https://books.google.com/books?id=AyXPQzEwqPIC&q=arthropod+species+number&pg=PA9
- Ruppert, Fox & Barnes (2004), pp. 518–522
- Schmidt-Nielsen, Knut (1984), "The strength of bones and skeletons", Scaling: Why is Animal Size So Important?, Cambridge University Press, pp. 42–55, ISBN 978-0-521-31987-4, https://books.google.com/books?id=8WkjD3L_avQC&q=arthropod+size+range&pg=PA53
- Williams, D.M. (April 21, 2001), "Largest", Book of Insect Records (University of Florida), http://entnemdept.ufl.edu/walker/ufbir/chapters/chapter_30.shtml, retrieved 2009-06-10
- Liu, Yu; Edgecombe, Gregory D.; Schmidt, Michel; Bond, Andrew D.; Melzer, Roland R.; Zhai, Dayou; Mai, Huijuan; Zhang, Maoyin et al. (2021-07-30). "Exites in Cambrian arthropods and homology of arthropod limb branches" (in en). Nature Communications 12 (1): 4619. doi:10.1038/s41467-021-24918-8. ISSN 2041-1723. PMID 34330912. Bibcode: 2021NatCo..12.4619L. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=8324779
- Gould (1990), pp. 102–106
- Ortega-Hernández, Javier; Janssen, Ralf; Budd, Graham E. (2017). "Origin and evolution of the panarthropod head – A palaeobiological and developmental perspective" (in en). Arthropod Structure & Development 46 (3): 354–379. doi:10.1016/j.asd.2016.10.011. PMID 27989966. https://linkinghub.elsevier.com/retrieve/pii/S1467803916301669.
- "Giant sea creature hints at early arthropod evolution". 2015-03-11. http://news.yale.edu/2015/03/11/giant-sea-creature-hints-early-arthropod-evolution.
- Fu, Dongjing; Legg, David A.; Daley, Allison C.; Budd, Graham E.; Wu, Yu; Zhang, Xingliang (2022-03-28). "The evolution of biramous appendages revealed by a carapace-bearing Cambrian arthropod". Philosophical Transactions of the Royal Society B: Biological Sciences 377 (1847): 20210034. doi:10.1098/rstb.2021.0034. PMID 35125000. https://dx.doi.org/10.1098%2Frstb.2021.0034
- Hejnol, Andreas; Scholtz, Gerhard (2004-10-01). "Clonal analysis of Distal-less and engrailed expression patterns during early morphogenesis of uniramous and biramous crustacean limbs" (in en). Development Genes and Evolution 214 (10): 473–485. doi:10.1007/s00427-004-0424-2. ISSN 1432-041X. PMID 15300435. https://doi.org/10.1007/s00427-004-0424-2.
- Wolff, Carsten; Scholtz, Gerhard (2008-05-07). "The clonal composition of biramous and uniramous arthropod limbs". Proceedings of the Royal Society B: Biological Sciences 275 (1638): 1023–1028. doi:10.1098/rspb.2007.1327. PMID 18252674. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2600901
- Shubin, Neil; Tabin, C.; Carroll, Sean (2000), "Fossils, Genes and the Evolution of Animal Limbs", in Gee, H., Shaking the Tree: Readings from Nature in the History of Life, University of Chicago Press, p. 110, ISBN 978-0-226-28497-2, https://books.google.com/books?id=M6yF0pU4eCsC&q=arthropod+diversity&pg=PA110
- Dunlop, Jason A.; Lamsdell, James C. (2017). "Segmentation and tagmosis in Chelicerata" (in en). Arthropod Structure & Development 46 (3): 395–418. doi:10.1016/j.asd.2016.05.002. PMID 27240897. https://linkinghub.elsevier.com/retrieve/pii/S1467803916300731.
- Whittington, H. B. (1971), "Redescription of Marrella splendens (Trilobitoidea) from the Burgess Shale, Middle Cambrian, British Columbia", Geological Survey of Canada Bulletin 209: 1–24 Summarised in Gould (1990), pp. 107–121.
- Budd, G. E. (16 May 2002), "A palaeontological solution to the arthropod head problem", Nature 417 (6886): 271–275, doi:10.1038/417271a, PMID 12015599, Bibcode: 2002Natur.417..271B https://dx.doi.org/10.1038%2F417271a
- "It would be too bad if the question of head segmentation ever should be finally settled; it has been for so long such fertile ground for theorizing that arthropodists would miss it as a field for mental exercise."[31]
- Wainwright, S. A.; Biggs, W. D.; Gosline, J. M. (1982), Mechanical Design in Organisms, Princeton University Press, pp. 162–163, ISBN 978-0-691-08308-7, https://archive.org/details/mechanicaldesign0000unse/page/162
- Lowenstam, H. A.; Weiner, S. (1989), On biomineralization, Oxford University Press, p. 111, ISBN 978-0-19-504977-0, https://books.google.com/books?id=JbAgy0AAopsC&q=arthropod+biomineralization
- Dzik, J (2007), "The Verdun Syndrome: simultaneous origin of protective armour and infaunal shelters at the Precambrian–Cambrian transition", in Vickers-Rich, Patricia; Komarower, Patricia, The Rise and Fall of the Ediacaran Biota, Special publications, 286, London: Geological Society, pp. 405–414, doi:10.1144/SP286.30, ISBN 9781862392335, OCLC 156823511, http://www.paleo.pan.pl/people/Dzik/Publications/Verdun.pdf
- Cohen, B. L. (2005), "Not armour, but biomechanics, ecological opportunity and increased fecundity as keys to the origin and expansion of the mineralized benthic metazoan fauna", Biological Journal of the Linnean Society 85 (4): 483–490, doi:10.1111/j.1095-8312.2005.00507.x, http://eprints.gla.ac.uk/2933/01/Cohen_2933.pdf, retrieved 2008-09-25
- Bengtson, S. (2004), Lipps, J. H.; Waggoner, B. M., eds., "Early skeletal fossils", The Paleontological Society Papers 10: neoproterozoic-cambrian biological revolutions: 67–78, doi:10.1017/S1089332600002345, http://www.starregister.org/download/18.4e32c81078a8d9249800021554/Bengtson2004ESF.pdf
- Barnes, R. S. K.; Calow, P.; Olive, P.; Golding, D.; Spicer, J. (2001), "Invertebrates with Legs: the Arthropods and Similar Groups", The Invertebrates: A Synthesis, Blackwell Publishing, p. 168, ISBN 978-0-632-04761-1, https://books.google.com/books?id=TBMsbe9efPgC&q=arthropod+hydraulic&pg=PA168
- Parry, D. A.; Brown, R. H. J. (1959), "The hydraulic mechanism of the spider leg", Journal of Experimental Biology 36 (2): 423–433, doi:10.1242/jeb.36.2.423, http://jeb.biologists.org/cgi/reprint/36/2/423.pdf, retrieved 2008-09-25
- Ruppert, Fox & Barnes (2004), pp. 523–524
- Ruppert, Fox & Barnes (2004), pp. 527–528
- error
- Ruppert, Fox & Barnes (2004), pp. 530, 733
- Ruppert, Fox & Barnes (2004), pp. 531–532
- Ruppert, Fox & Barnes (2004), pp. 529–530
- Ruppert, Fox & Barnes (2004), pp. 532–537
- Ruppert, Fox & Barnes (2004), pp. 578–580
- Völkel, R.; Eisner, M.; Weible, K. J. (June 2003). "Miniaturized imaging systems". Microelectronic Engineering 67–68: 461–472. doi:10.1016/S0167-9317(03)00102-3. http://www.suss-microoptics.com/downloads/Publications/Miniaturized_Imaging_Systems.pdf.
- Ruppert, Fox & Barnes (2004), pp. 537–539
- Olive, P. J. W. (2001). "Encyclopedia of Life Sciences". Encyclopedia of Life Sciences. John Wiley & Sons. doi:10.1038/npg.els.0003649. ISBN 978-0470016176. https://dx.doi.org/10.1038%2Fnpg.els.0003649
- Schurko, A. M.; Mazur, D. J.; Logsdon, J. M. (February 2010). "Inventory and phylogenomic distribution of meiotic genes in Nasonia vitripennis and among diverse arthropods". Insect Molecular Biology 19 Suppl 1: 165–180. doi:10.1111/j.1365-2583.2009.00948.x. PMID 20167026. https://dx.doi.org/10.1111%2Fj.1365-2583.2009.00948.x
- Bernstein, H.; Hopf, F. A.; Michod, R. E. (1987). "The molecular basis of the evolution of sex". Advances in Genetics 24: 323–370. doi:10.1016/s0065-2660(08)60012-7. ISBN 9780120176243. PMID 3324702. https://dx.doi.org/10.1016%2Fs0065-2660%2808%2960012-7
- Lourenço, W. R. (2002), "Reproduction in scorpions, with special reference to parthenogenesis", in Toft, S.; Scharff, N., European Arachnology 2000, Aarhus University Press, pp. 71–85, ISBN 978-87-7934-001-5, http://www.european-arachnology.org/proceedings/19th/Lourenco.PDF, retrieved 2008-09-28
- Truman, J. W.; Riddiford, L. M. (September 1999), "The origins of insect metamorphosis", Nature 401 (6752): 447–452, doi:10.1038/46737, PMID 10519548, Bibcode: 1999Natur.401..447T, http://www.insecta.ufv.br/Entomologia/ent/disciplina/ban%20160/AULAT/aula8/truman.pdf, retrieved 2008-09-28
- Smith, G., Diversity and Adaptations of the Aquatic Insects, New College of Florida, http://faculty.ncf.edu/mccord/pdf/AquaticInsectGeoffSmith.pdf, retrieved 2008-09-28
- Bergström, Jan; Hou, Xian-Guang (2005), "Early Palaeozoic non-lamellipedian arthropods", in Stefan Koenemann; Ronald A. Jenner, Crustacea and Arthropod Relationships, Crustacean Issues, 16, Boca Raton: Taylor & Francis, pp. 73–93, doi:10.1201/9781420037548.ch4, ISBN 978-0-8493-3498-6 https://dx.doi.org/10.1201%2F9781420037548.ch4
- "Arthropod ancestor had the mouth of a penis worm - Natural History Museum". http://www.nhm.ac.uk/our-science/science-news/2016/september/arthropod-ancestor-mouth.html.
- Glaessner, M. F. (1958). "New fossils from the base of the Cambrian in South Australia". Transactions of the Royal Society of South Australia 81: 185–188. http://www.samuseum.sa.gov.au/Journals/TRSSA/TRSSA_V081/TRSSA_V081_p185p188.pdf.
- Lin, J. P.; Gon, S. M.; Gehling, J. G.; Babcock, L. E.; Zhao, Y. L.; Zhang, X. L.; Hu, S. X.; Yuan, J. L. et al. (2006). "A Parvancorina-like arthropod from the Cambrian of South China". Historical Biology 18 (1): 33–45. doi:10.1080/08912960500508689. https://dx.doi.org/10.1080%2F08912960500508689
- McMenamin, M.A.S (2003), "Spriggina is a trilobitoid ecdysozoan" (abstract), Abstracts with Programs 35 (6): 105, http://gsa.confex.com/gsa/2003AM/finalprogram/abstract_62056.htm, retrieved 2008-10-21
- Daley, Allison C.; Antcliffe, Jonathan B.; Drage, Harriet B.; Pates, Stephen (2018-05-22). "Early fossil record of Euarthropoda and the Cambrian Explosion". Proceedings of the National Academy of Sciences of the United States of America 115 (21): 5323–5331. doi:10.1073/pnas.1719962115. ISSN 0027-8424. PMID 29784780. Bibcode: 2018PNAS..115.5323D. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6003487
- Braun, A.; J. Chen; D. Waloszek; A. Maas (2007), "First Early Cambrian Radiolaria", Special Publications 286 (1): 143–149, doi:10.1144/SP286.10, Bibcode: 2007GSLSP.286..143B, http://biosys-serv.biologie.uni-ulm.de/Downloadfolder/PDFs%20Team/2007b_Braun_etal.pdf
- 2.0.CO;2. ISSN 0091-7613. Bibcode: 2002Geo....30..363Y. https://www.researchgate.net/publication/249520833. " id="ref_57">Yuan, X.; Xiao, S.; Parsley, R. L.; Zhou, C.; Chen, Z.; Hu, J. (April 2002). "Towering sponges in an Early Cambrian Lagerstätte: Disparity between nonbilaterian and bilaterian epifaunal tierers at the Neoproterozoic-Cambrian transition". Geology 30 (4): 363–366. doi:10.1130/0091-7613(2002)030<0363:TSIAEC>2.0.CO;2. ISSN 0091-7613. Bibcode: 2002Geo....30..363Y. https://www.researchgate.net/publication/249520833.
- Skovsted, Christian; Brock, Glenn; Paterson, John (2006), "Bivalved arthropods from the Lower Cambrian Mernmerna Formation of South Australia and their implications for the identification of Cambrian 'small shelly fossils'", Association of Australasian Palaeontologists Memoirs 32: 7–41, ISSN 0810-8889, https://www.researchgate.net/publication/303809761
- Betts, Marissa; Topper, Timothy; Valentine, James; Skovsted, Christian; Paterson, John; Brock, Glenn (January 2014), "A new early Cambrian bradoriid (Arthropoda) assemblage from the northern Flinders Ranges, South Australia", Gondwana Research 25 (1): 420–437, doi:10.1016/j.gr.2013.05.007, Bibcode: 2014GondR..25..420B, https://www.researchgate.net/publication/236901156
- Lieberman, B. S. (March 1, 1999), "Testing the Darwinian legacy of the Cambrian radiation using trilobite phylogeny and biogeography", Journal of Paleontology 73 (2): 176, doi:10.1017/S0022336000027700, http://jpaleontol.geoscienceworld.org/cgi/content/abstract/73/2/176, retrieved October 21, 2008
- error
- Fu, D.; Legg, D. A.; Daley, A. C.; Budd, G. E.; Wu, Y.; Zhang, X. (2022). "The evolution of biramous appendages revealed by a carapace-bearing Cambrian arthropod". Philosophical Transactions of the Royal Society B: Biological Sciences 377 (1847): Article ID 20210034. doi:10.1098/rstb.2021.0034. PMID 35125000. https://dx.doi.org/10.1098%2Frstb.2021.0034
- Whittington, H. B. (1979). Early arthropods, their appendages and relationships. In M. R. House (Ed.), The origin of major invertebrate groups (pp. 253–268). The Systematics Association Special Volume, 12. London: Academic Press.
- Whittington, H.B.; Geological Survey of Canada (1985), The Burgess Shale, Yale University Press, ISBN 978-0-660-11901-4, OCLC 15630217 http://www.worldcat.org/oclc/15630217
- Gould (1990)
- García-Bellido, D. C.; Collins, D. H. (May 2004), "Moulting arthropod caught in the act", Nature 429 (6987): 40, doi:10.1038/429040a, PMID 15129272, Bibcode: 2004Natur.429...40G https://dx.doi.org/10.1038%2F429040a
- Budd, G. E.; Butterfield, N. J.; Jensen, S. (December 2001), "Crustaceans and the "Cambrian Explosion"", Science 294 (5549): 2047, doi:10.1126/science.294.5549.2047a, PMID 11739918 https://dx.doi.org/10.1126%2Fscience.294.5549.2047a
- Callaway, E. (9 October 2008), Fossilised shrimp show earliest group behaviour, New Scientist, https://www.newscientist.com/channel/life/dn14903-fossilised-shrimp-show-earliest-group-behaviour.html, retrieved 2008-10-21
- Pisani, D.; Poling, L. L.; Lyons-Weiler M.; Hedges, S. B. (2004), "The colonization of land by animals: molecular phylogeny and divergence times among arthropods", BMC Biology 2: 1, doi:10.1186/1741-7007-2-1, PMID 14731304 http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=333434
- Cowen, R. (2000), History of Life (3rd ed.), Blackwell Science, p. 126, ISBN 978-0-632-04444-3
- Braddy, S. J.; Markus Poschmann, M.; Tetlie, O. E. (2008), "Giant claw reveals the largest ever arthropod", Biology Letters 4 (1): 106–109, doi:10.1098/rsbl.2007.0491, PMID 18029297 http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2412931
- Dunlop, J. A. (September 1996), "A trigonotarbid arachnid from the Upper Silurian of Shropshire", Palaeontology 39 (3): 605–614, http://palaeontology.palass-pubs.org/pdf/Vol%2039/Pages%20605-614.pdf
- The fossil was originally named Eotarbus but was renamed when it was realized that a Carboniferous arachnid had already been named Eotarbus.[78]
- Selden, P. A.; Shear, W. A. (December 2008), "Fossil evidence for the origin of spider spinnerets", PNAS 105 (52): 20781–5, doi:10.1073/pnas.0809174106, PMID 19104044, Bibcode: 2008PNAS..10520781S http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2634869
- Selden, P. A. (February 1996), "Fossil mesothele spiders", Nature 379 (6565): 498–499, doi:10.1038/379498b0, Bibcode: 1996Natur.379..498S https://dx.doi.org/10.1038%2F379498b0
- Vollrath, F.; Selden, P. A. (December 2007), "The Role of Behavior in the Evolution of Spiders, Silks, and Webs", Annual Review of Ecology, Evolution, and Systematics 38: 819–846, doi:10.1146/annurev.ecolsys.37.091305.110221, http://homepage.mac.com/paulselden/Sites/Website/ARES.pdf
- Jeram, A. J. (January 1990), "Book-lungs in a Lower Carboniferous scorpion", Nature 343 (6256): 360–361, doi:10.1038/343360a0, Bibcode: 1990Natur.343..360J https://dx.doi.org/10.1038%2F343360a0
- Engel, M. S.; Grimaldi, D. A. (February 2004), "New light shed on the oldest insect", Nature 427 (6975): 627–630, doi:10.1038/nature02291, PMID 14961119, Bibcode: 2004Natur.427..627E https://dx.doi.org/10.1038%2Fnature02291
- Haug, Carolin; Haug, Joachim T. (2017-05-30). "The presumed oldest flying insect: more likely a myriapod?". PeerJ 5: e3402. doi:10.7717/peerj.3402. ISSN 2167-8359. PMID 28584727. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5452959
- Labandeira, C.; Eble, G. J. (2000), "The Fossil Record of Insect Diversity and Disparity", in Anderson, J.; Thackeray, F.; van Wyk, B. et al., Gondwana Alive: Biodiversity and the Evolving Biosphere, Witwatersrand University Press, http://www.santafe.edu/research/publications/workingpapers/00-08-044.pdf, retrieved 2008-10-21
- Budd, G. E. (1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia 29 (1): 1–14. doi:10.1111/j.1502-3931.1996.tb01831.x. https://dx.doi.org/10.1111%2Fj.1502-3931.1996.tb01831.x
- Gillott, C. (1995), Entomology, Springer, pp. 17–19, ISBN 978-0-306-44967-3
- Adrain, J. (15 March 1999), Book Review: Arthropod Fossils and Phylogeny, edited by Gregory D. Edgecomb, Palaeontologia Electronica, http://palaeo-electronica.org/1999_1/books/arthropo.htm, retrieved 2008-09-28 The book is Labandiera, Conrad C.; Edgecombe, Gregory (1998), G. D., ed., "Arthropod Fossils and Phylogeny", PALAIOS (Columbia University Press) 14 (4): 347, doi:10.2307/3515467, Bibcode: 1999Palai..14..405L
- Chen, J.-Y.; Edgecombe, G. D.; Ramsköld, L.; Zhou, G.-Q. (2 June 1995), "Head segmentation in Early Cambrian Fuxianhuia: implications for arthropod evolution", Science 268 (5215): 1339–1343, doi:10.1126/science.268.5215.1339, PMID 17778981, Bibcode: 1995Sci...268.1339C https://dx.doi.org/10.1126%2Fscience.268.5215.1339
- Budd, G. E. (1993). "A Cambrian gilled lobopod from Greenland". Nature 364 (6439): 709–711. doi:10.1038/364709a0. Bibcode: 1993Natur.364..709B. https://dx.doi.org/10.1038%2F364709a0
- Nielsen, C. (2001). Animal Evolution: Interrelationships of the Living Phyla (2nd ed.). Oxford University Press. pp. 194–196. ISBN 978-0-19-850681-2. https://books.google.com/books?id=UmCg6c0HkqMC&q=nielsen+panarthropoda+euarthropoda&pg=PA194.
- Hou, X.-G.; Bergström, J.; Jie, Y. (2006), "Distinguishing anomalocaridids from arthropods and priapulids", Geological Journal 41 (3–4): 259–269, doi:10.1002/gj.1050 https://dx.doi.org/10.1002%2Fgj.1050
- "Misunderstood worm-like fossil finds its place in the Tree of Life". 17 August 2014. http://www.cam.ac.uk/research/news/misunderstood-worm-like-fossil-finds-its-place-in-the-tree-of-life.
- Telford, M. J.; Bourlat, S. J.; Economou, A.; Papillon, D.; Rota-Stabelli, O. (January 2008). "The evolution of the Ecdysozoa". Philosophical Transactions of the Royal Society B: Biological Sciences 363 (1496): 1529–1537. doi:10.1098/rstb.2007.2243. PMID 18192181. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2614232
- Vaccari, N. E.; Edgecombe, G. D.; Escudero, C. (29 July 2004), "Cambrian origins and affinities of an enigmatic fossil group of arthropods", Nature 430 (6999): 554–557, doi:10.1038/nature02705, PMID 15282604, Bibcode: 2004Natur.430..554V https://dx.doi.org/10.1038%2Fnature02705
- 3.0.CO;2-L. PMID 29852696. https://dx.doi.org/10.1002%2F%28SICI%291097-4687%28199812%29238%3A3%3C263%3A%3AAID-JMOR1%3E3.0.CO%3B2-L" id="ref_91">Schmidt-Rhaesa, A.; Bartolomaeus, T.; Lemburg, C.; Ehlers, U.; Garey, J. R. (January 1999). "The position of the Arthropoda in the phylogenetic system". Journal of Morphology 238 (3): 263–285. doi:10.1002/(SICI)1097-4687(199812)238:3<263::AID-JMOR1>3.0.CO;2-L. PMID 29852696. https://dx.doi.org/10.1002%2F%28SICI%291097-4687%28199812%29238%3A3%3C263%3A%3AAID-JMOR1%3E3.0.CO%3B2-L
- Ortega-Hernández, Javier (2016), "Making sense of 'lower' and 'upper' stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848", Biol. Rev. 91 (1): 255–273, doi:10.1111/brv.12168, PMID 25528950 https://dx.doi.org/10.1111%2Fbrv.12168
- Gregory D. Edgecombe (2020), "Arthropod Origins: Integrating Paleontological and Molecular Evidence", Annu. Rev. Ecol. Evol. Syst. 51: 1–25, doi:10.1146/annurev-ecolsys-011720-124437 https://dx.doi.org/10.1146%2Fannurev-ecolsys-011720-124437
- Zeng, Han; Zhao, Fangchen; Niu, Kecheng; Zhu, Maoyan; Huang, Diying (November 2020), "An early Cambrian euarthropod with radiodont-like raptorial appendages", Nature 588 (7836): 101–105, doi:10.1038/s41586-020-2883-7, PMID 33149303, Bibcode: 2020Natur.588..101Z https://dx.doi.org/10.1038%2Fs41586-020-2883-7
- O’Flynn, Robert; Williams, Mark; Yu, Mengxiao; Harvey, Thomas; Liu, Yu (2022), "A new euarthropod with large frontal appendages from the early Cambrian Chengjiang biota", Palaeontologia Electronica 25 (1): a6, doi:10.26879/1167 https://dx.doi.org/10.26879%2F1167
- Aria, Cédric; Caron, Jean-Bernard (April 2017), "Burgess Shale fossils illustrate the origin of the mandibulate body plan", Nature 545 (7652): 89–92, doi:10.1038/nature22080, PMID 28445464, Bibcode: 2017Natur.545...89A https://dx.doi.org/10.1038%2Fnature22080
- Lozano-Fernandez, Jesus; Giacomelli, Mattia; F. Fleming, James; Chen, Albert; Vinther, Jakob; Thomsen, Philip Francis; Glenner, Henrik; Palero, Ferran et al. (2019), "Pancrustacean Evolution Illuminated by Taxon-Rich GenomicScale Data Sets with an Expanded Remipede Sampling", Genome Biol. Evol. 11 (8): 2055–2070, doi:10.1093/gbe/evz097, PMID 31270537 http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6684935
- Giribet, Gonzalo; Edgecombe, Gregory (June 2019), "The Phylogeny and Evolutionary History of Arthropods", Current Biology 29 (12): R592–R602, doi:10.1016/j.cub.2019.04.057, PMID 31211983 https://dx.doi.org/10.1016%2Fj.cub.2019.04.057
- "Phylogenomics resolves the timing and pattern of insect evolution", Science 346 (6210): 763–767, 2014, doi:10.1126/science.1257570, PMID 25378627, Bibcode: 2014Sci...346..763M https://dx.doi.org/10.1126%2Fscience.1257570
- Lua error: Internal error: The interpreter exited with status 1.
- Carapelli, Antonio; Liò, Pietro; Nardi, Francesco; van der Wath, Elizabeth; Frati, Francesco (16 August 2007). "Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea". BMC Evolutionary Biology 7 (Suppl 2): S8. doi:10.1186/1471-2148-7-S2-S8. PMID 17767736. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1963475
- Regier, Jerome C. et al. (2010). "Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences". Nature 463 (7284): 1079–1084. doi:10.1038/nature08742. PMID 20147900. Bibcode: 2010Natur.463.1079R. https://dx.doi.org/10.1038%2Fnature08742
- von Reumont, Bjoern M.; Jenner, Ronald A.; Wills, Matthew A.; Dell’Ampio, Emiliano; Pass, Günther; Ebersberger, Ingo; Meyer, Benjamin; Koenemann, Stefan et al. (2011), "Pancrustacean phylogeny in the light of new phylogenomic data: support for Remipedia as the possible sister group of Hexapoda", Molecular Biology and Evolution 29 (3): 1031–45, doi:10.1093/molbev/msr270, PMID 22049065 https://dx.doi.org/10.1093%2Fmolbev%2Fmsr270
- Hassanin, Alexandre (2006). "Phylogeny of Arthropoda inferred from mitochondrial sequences: Strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution". Molecular Phylogenetics and Evolution 38 (1): 100–116. doi:10.1016/j.ympev.2005.09.012. PMID 16290034. http://www.csulb.edu/~dlunderw/entomology/Arthropodphylogeny2006.pdf. Retrieved 2010-04-16.
- Giribet, G.; Richter, S.; Edgecombe, G. D.; Wheeler, W. C. (2005). The position of crustaceans within Arthropoda – Evidence from nine molecular loci and morphology. Crustacean Issues. 16. pp. 307–352. doi:10.1201/9781420037548.ch13. ISBN 978-0-8493-3498-6. http://www.amonline.net.au/palaeontology/pdf/giribet-etal2005.pdf. Retrieved 2006-08-23.
- Jenner, R. A. (April 2006). "Challenging received wisdoms: Some contributions of the new microscopy to the new animal phylogeny". Integrative and Comparative Biology 46 (2): 93–103. doi:10.1093/icb/icj014. PMID 21672726. https://dx.doi.org/10.1093%2Ficb%2Ficj014
- Dunlop, Jason A. (31 January 2011), "Fossil Focus: Chelicerata", PALAEONTOLOGY[online], http://www.palaeontologyonline.com/articles/2011/fossil-focus-chelicerata/, retrieved 15 March 2018
- "Arthropoda". http://www.peripatus.gen.nz/Taxa/Arthropoda/Index.html.
- Wickins, J. F.; Lee, D. O'C. (2002). Crustacean Farming: Ranching and Culture (2nd ed.). Blackwell. ISBN 978-0-632-05464-0. http://www.blackwellpublishing.com/book.asp?ref=9780632054640. Retrieved 2008-10-03.
- Bailey, S., Bugfood II: Insects as Food!?!, University of Kentucky Department of Entomology, http://www.uky.edu/Ag/Entomology/ythfacts/bugfood/bugfood2.htm, retrieved 2008-10-03
- Unger, L., Bugfood III: Insect Snacks from Around the World, University of Kentucky Department of Entomology, http://www.uky.edu/Ag/Entomology/ythfacts/bugfood/yf813.htm, retrieved 2008-10-03
- Rigby, R. (September 21, 2002), "Tuck into a Tarantula", Sunday Telegraph, http://www.rhymer.net/cutsE.htm, retrieved 2009-08-24
- Spiderwomen serve up Cambodia's creepy caviar, ABC News Online, September 2, 2002, http://abc.net.au/news/indepth/featureitems/s664704.htm, retrieved 2009-08-24
- Ray, N. (2002). Lonely Planet Cambodia. Lonely Planet Publications. p. 308. ISBN 978-1-74059-111-9.
- Weil, C. (2006), Fierce Food, Plume, ISBN 978-0-452-28700-6, https://archive.org/details/fiercefoodintrep0000weil, retrieved 2008-10-03
- Taylor, R. L. (1975), Butterflies in My Stomach (or: Insects in Human Nutrition), Woodbridge Press Publishing Company, Santa Barbara, California
- For a mention of insect contamination in an international food quality standard, see sections 3.1.2 and 3.1.3 of Codex 152 of 1985 of the Codex Alimentarius[121]
- For examples of quantified acceptable insect contamination levels in food see the last entry (on "Wheat Flour") and the definition of "Extraneous material" in Codex Alimentarius,[122] and the standards published by the FDA.[123]
- Paoletti, M. G. (2005), Ecological implications of minilivestock: potential of insects, rodents, frogs, and snails, Science Publishers, pp. 648, ISBN 978-1-57808-339-8, https://books.google.com/books?id=u4eTQgAACAAJ
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B. E. (August 2008). "Economic valuation of the vulnerability of world agriculture confronted with pollinator decline". Ecological Economics 68 (3): 810–821. doi:10.1016/j.ecolecon.2008.06.014. https://halshs.archives-ouvertes.fr/halshs-01293686/file/Gallai%20et%20al.%202009%20Ecological%20Economics%20Economic%20Valiuation%20of%20Poll.pdf. Retrieved 2018-11-24. Free summary at Gallai, N.; Salles, J.; Settele, J.; Vaissiere, B. (2009), "Economic value of insect pollination worldwide estimated at 153 billion euros", Ecological Economics 68 (3): 810–821, doi:10.1016/j.ecolecon.2008.06.014, http://www.eurekalert.org/pub_releases/2008-09/haog-evo091508.php, retrieved 2008-10-03
- Apiservices — International honey market — World honey production, imports & exports, http://www.beekeeping.com/databases/honey-market/world_honey.htm, retrieved 2008-10-03
- Threads In Tyme, LTD, Time line of fabrics, http://threadsintyme.tripod.com/id63.htm, retrieved 2005-07-14
- Jeff Behan, The bug that changed history, http://www.gcrg.org/bqr/8-2/bug.htm, retrieved 2006-06-26
- Canary Islands cochineal producers homepage, http://www.arrakis.es/~rpdeblas/cochinea.htm, retrieved 2005-07-14
- Heard, W., Coast, University of South Florida, http://www.marine.usf.edu/pjocean/packets/f01/f01u5p3.pdf, retrieved 2008-08-25
- Hall, R. D.; Castner, J. L. (2000), "Introduction", in Byrd, J. H.; Castner, J. L., Forensic Entomology: the Utility of Arthropods in Legal Investigations, CRC Press, pp. 3–4, ISBN 978-0-8493-8120-1, https://books.google.com/books?id=iAtRGA2IfcwC&pg=PA3
- Dossey, Aaron (December 2010), "Insects and their chemical weaponry: New potential for drug discovery", Natural Product Reports 27 (12): 1737–1757, doi:10.1039/C005319H, PMID 20957283 https://dx.doi.org/10.1039%2FC005319H
- Spagna, J. C.; Goldman D. I.; Lin P.-C.; Koditschek D. E.; R. J. Full (March 2007), "Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain", Bioinspiration & Biomimetics 2 (1): 9–18, doi:10.1088/1748-3182/2/1/002, PMID 17671322, Bibcode: 2007BiBi....2....9S, http://polypedal.berkeley.edu/twiki/pub/PolyPEDAL/PolypedalPublications/Distributed_BB.pdf
- Kazuo Tsuchiya; Shinya Aoi; Katsuyoshi Tsujita (2006), "A Turning Strategy of a Multi-legged Locomotion Robot", Adaptive Motion of Animals and Machines, pp. 227–236, doi:10.1007/4-431-31381-8_20, ISBN 978-4-431-24164-5 https://dx.doi.org/10.1007%2F4-431-31381-8_20
- Hill, D. (1997), The Economic Importance of Insects, Springer, pp. 77–92, ISBN 978-0-412-49800-8
- Goodman, Jesse L.; Dennis, David Tappen; Sonenshine, Daniel E. (2005), Tick-borne diseases of humans, ASM Press, p. 114, ISBN 978-1-55581-238-6, https://books.google.com/books?id=dKlUARLKT9IC, retrieved 2010-03-29
- Potter, M. F., Parasitic Mites of Humans, University of Kentucky College of Agriculture, http://www.ca.uky.edu/entomology/entfacts/ef637.asp, retrieved 2008-10-25
- Klenerman, Paul; Lipworth, Brian; authors, House dust mite allergy, NetDoctor, http://www.netdoctor.co.uk/health_advice/facts/allergyhousedustmite.htm, retrieved 2008-02-20
- Kogan, M.; Croft, B. A.; Sutherst, R. F. (1999), "Applications of ecology for integrated pest management", in Huffaker, Carl B.; Gutierrez, A. P., Ecological Entomology, John Wiley & Sons, pp. 681–736, ISBN 978-0-471-24483-7, https://books.google.com/books?id=aw5Iycas70cC&pg=PA681
- Gorham, J. Richard (1991), "Insect and Mite Pests in Food : An Illustrated Key", Agriculture Handbook Number 655 (United States Department of Agriculture): pp. 1–767, http://www.afpmb.org/pubs/tims/tg27/docs/Insect%20and%20Mite%20Pests%20in%20Food%20Gorham.pdf, retrieved 2010-05-06
- Jong, D. D.; Morse, R. A.; Eickwort, G. C. (January 1982), "Mite Pests of Honey Bees", Annual Review of Entomology 27: 229–252, doi:10.1146/annurev.en.27.010182.001305 https://dx.doi.org/10.1146%2Fannurev.en.27.010182.001305
- Metcalf, Robert Lee; Luckmann, William Henry (1994), Introduction to insect pest management, Wiley-IEEE, p. 4, ISBN 978-0-471-58957-0, https://books.google.com/books?id=pW1dXL2EgnMC
- Shultz, J. W. (2001), "Chelicerata (Arachnids, Including Spiders, Mites and Scorpions)", Encyclopedia of Life Sciences, John Wiley & Sons, Ltd., doi:10.1038/npg.els.0001605, ISBN 978-0470016176 https://dx.doi.org/10.1038%2Fnpg.els.0001605
- Osakabe, M. (March 2002), "Which predatory mite can control both a dominant mite pest, Tetranychus urticae, and a latent mite pest, Eotetranychus asiaticus, on strawberry?", Experimental and Applied Acarology 26 (3–4): 219–230, doi:10.1023/A:1021116121604, PMID 12542009 https://dx.doi.org/10.1023%2FA%3A1021116121604
- He, Xueqing; Kiær, Lars Pødenphant; Jensen, Per Moestrup; Sigsgaard, Lene (2021). "The effect of floral resources on predator longevity and fecundity: A systematic review and meta-analysis". Biological Control (Elsevier BV) 153: 104476. doi:10.1016/j.biocontrol.2020.104476. ISSN 1049-9644. https://dx.doi.org/10.1016%2Fj.biocontrol.2020.104476
- He, Xueqing; Sigsgaard, Lene (2019-02-05). "A Floral Diet Increases the Longevity of the Coccinellid Adalia bipunctata but Does Not Allow Molting or Reproduction". Frontiers in Ecology and Evolution (Frontiers Media SA) 7. doi:10.3389/fevo.2019.00006. ISSN 2296-701X. https://dx.doi.org/10.3389%2Ffevo.2019.00006