The problem with increasing the yield of recombinant proteins is resolvable using different approaches, including the transport of a target protein to cell compartments with a low protease activity. In the cell, protein targeting involves short-signal peptide sequences recognized by intracellular protein transport systems.
- recombinant protein
- transport signal peptide
- plant expression system
1. Introduction
Mitochondria and plastids are endosymbiotic organelles originating from ancestors of extant α-proteobacteria and cyanobacteria. The symbiosis commenced at different time points; the progenitors of mitochondria were the first to be taken up by a proto-eukaryotic cell, followed by plastids [1]. The transformation of endosymbionts into organelles has been accompanied by the massive translocation of their genetic material to the nucleus. Given that most of mitochondrial and plastid proteins are synthesized in the cytosol on nuclear transcripts [2][3][4], these proteins must be targeted from the cytosol to the corresponding organelles. The majority of proteins targeted to plastids and mitochondria carry at their N terminus an SP named the “transit peptide” for chloroplasts and “presequence” for mitochondrial targeting; it is recognized by import mechanisms of these organelles and then cleaved by specific signal peptidases. One of the most intriguing aspects is that the protein-targeting mechanisms of plastids and mitochondria are very similar [5]. Along with the specific sequences targeting proteins to either plastids or mitochondria, some SPs are recognizable by the import machineries of both organelles [6]. Recently, an ever-increasing volume of data revealed that proteins are targeted to mitochondria or to the ER not only by means of SPs but also at the level of the targeted transport of the mRNAs carrying zip codes for the interaction with RNA-binding proteins at their 3′ end [7][8]. As a result, the mRNAs and even mRNA complexes with ribosomes are localized near the mitochondrial outer membrane and even on its surface, thereby directly interacting with its import machinery. Note that protein targeting to mitochondria can be not only post-translational, as previously believed, but also cotranslational. The role of mRNA targeting in the case of plastids is rather vague; however, some indirect evidence suggests that it occurs there too [9][10].
2. Major Mechanisms Underlying Protein Import into Mitochondria
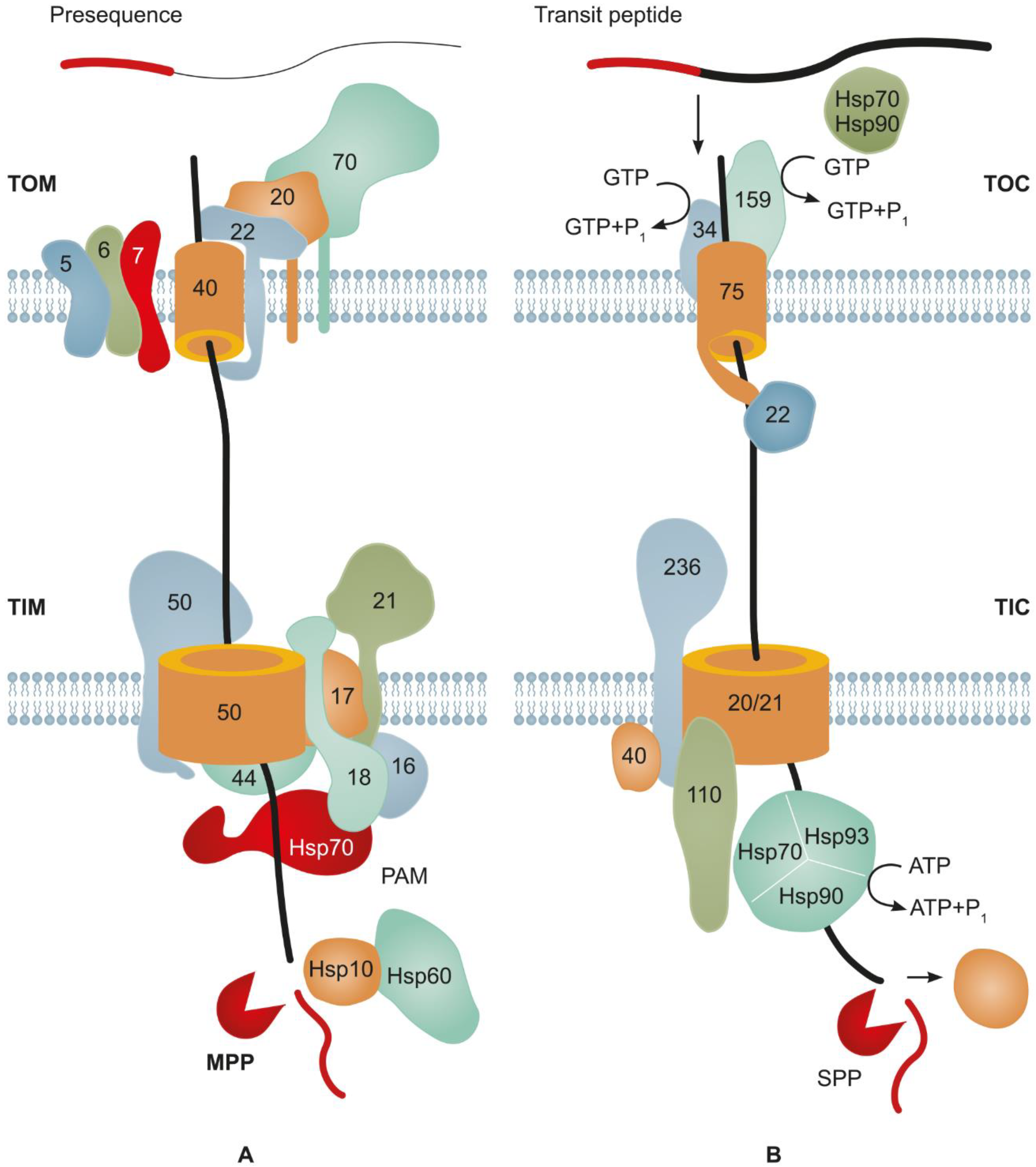
3. Main Mechanisms of Protein Import into Plastids
4. Structure of SPs Targeting Proteins to Endosymbiotic Organelles

5. Targeting of Recombinant Proteins to Endosymbiotic Organelles
This entry is adapted from the peer-reviewed paper 10.3390/plants11192561
References
- Margulis, L. Symbiosis in Cell Evolution: Life and Its Environment on the Early Earth; Freeman Press: San Francisco, CA, USA, 1981.
- Wiedemann, N.; Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 2017, 86, 685–714.
- Dobrogojski, J.; Adamiec, M.; Luciński, R. The chloroplast genome: A review. Acta Physiol. Plant. 2020, 42, 98.
- Zoschke, R.; Bock, R. Chloroplast translation: Structural and functional organization, operational control, and regulation. Plant Cell 2018, 30, 745–770.
- Schleiff, E.; Becker, T. Common ground for protein translocation: Access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 2011, 12, 48–59.
- Ge, C.; Spenning, E.; Glaser, E.; Wieslander, E. Import determinants of organelle-specific and dual targeting peptides of mitochondria and chloroplasts in Arabidopsis thaliana. Mol. Plant. 2014, 7, 121–136.
- Yogev, O.; Karniely, S.; Pines, O. Translation-coupled translocation of yeast fumarase into mitochondria in vivo. J. Biol. Chem. 2007, 282, 29222–29229.
- Eliyahu, E.; Pnueli, L.; Melamed, D.; Scherrer, T.; Gerber, A.P.; Pines, O.; Rapaport, D.; Arava, Y. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. Mol. Cell. Biol. 2010, 30, 284–294.
- Weis, B.L.; Schleiff, E.; Zerges, W. Protein targeting to subcellular organelles via MRNA localization. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 260–273.
- Lashkevich, K.A.; Dmitriev, S.E. mRNA targeting, transport and local translation in eukaryotic cells: From the classical view to a diversity of new concepts. Mol. Biol. 2021, 55, 507–537.
- Maity, S.; Chakrabarti, O. Mitochondrial protein import as a quality control sensor. Biol. Cell 2021, 113, 375–400.
- Tucker, K.; Park, E. Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution. Nat. Struct. Mol. Biol. 2019, 26, 1158–1166.
- Edwards, R.; Gerlich, S.; Tokatlidis, K. The biogenesis of mitochondrial intermembrane space proteins. Biol. Chem. 2020, 401, 737–747.
- Becker, T.; Song, J.; Pfanner, N. Versatility of preprotein transfer from the cytosol to mitochondria. Trends Cell. Biol. 2019, 29, 534–548.
- Bykov, Y.S.; Rapaport, D.; Herrmann, J.M.; Schuldiner, M. Cytosolic events in the biogenesis of mitochondrial proteins. Trends Biochem. Sci. 2020, 45, 650–667.
- Backes, S.; Bykov, Y.S.; Flohr, T.; Räschle, M.; Zhou, J.; Lenhard, S.; Krämer, L.; Mühlhaus, T.; Bibi, C.; Jann, C.; et al. The chaperone-binding activity of the mitochondrial surface receptor Tom70 protects the cytosol against mitoprotein-induced stress. Cell Rep. 2021, 35, 108936.
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354.
- Richardson, L.G.L.; Small, E.L.; Inoue, H.; Schnell, D.J. Molecular topology of the transit peptide during chloroplast protein import. Plant Cell 2018, 30, 1789–1806.
- Craig, E.A.; Marszalek, J. How do J-proteins get Hsp70 to do so many different things? Trends Biochem. Sci. 2017, 42, 355–368.
- Opalinski, L.; Song, J.; Priesnitz, C.; Wenz, L.S.; Oeljeklaus, S.; Warscheid, B.; Pfanner, N.; Becker, T. Recruitment of cytosolic J-proteins by TOM receptors promotes mitochondrial protein biogenesis. Cell Rep. 2018, 25, 2036–2043.
- Hansen, K.G.; Aviram, N.; Laborenz, J.; Bibi, C.; Meyer, M.; Spang, A.; Schuldiner, M.; Herrmann, J.M. An ER surface retrieval pathway safeguards the import of mitochondrial membrane proteins in yeast. Science 2018, 361, 1118–1122.
- Gold, V.A.; Chroscicki, P.; Bragoszewski, P.; Chacinska, A. Visualization of cytosolic ribosomes on the surface of mitochondria by electron cryo-tomography. EMBO Rep. 2017, 18, 1786–1800.
- Williams, C.C.; Jan, C.H.; Weissman, J.S. Targeting and plasticity of mitochondrial proteins revealed by proximity-specific ribosome profiling. Science 2014, 346, 748–751.
- Marc, P.; Margeot, A.; Devaux, F.; Blugeon, C.; Corral-Debrinski, M.; Jacq, C. Genome-wide analysis of mRNAs targeted to yeast mitochondria. EMBO Rep. 2002, 3, 159–164.
- Lapointe, C.P.; Stefely, J.A.; Jochem, A.; Hutchins, P.D.; Wilson, G.M.; Kwiecien, N.W.; Coon, J.J.; Wickens, M.; Pagliarini, D.J. Multi-omics reveal specific targets of the RNA-binding protein Puf3p and its orchestration of mitochondrial biogenesis. Cell Syst. 2018, 6, 125–135.
- Saint-Georges, Y.; Garcia, M.; Delaveau, T.; Jourdren, L.; Le Crom, S.; Lemoine, S.; Tanty, V.; Devaux, F.; Jacq, C. Yeast mitochondrial biogenesis: A role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS ONE 2008, 3, e2293.
- Møller, I.M.; Rasmusson, A.G.; Van Aken, O. Plant mitochondria–past, present and future. Plant J. 2021, 108, 912–959.
- Lesnik, C.; Cohen, Y.; Atir-Lande, A.; Schuldiner, M.; Arava, Y. OM14 is a mitochondrial receptor for cytosolic ribosomes that supports co-translational import into mitochondria. Nat. Commun. 2014, 5, 5711.
- Ponce-Rojas, J.C.; Avendaño-Monsalve, M.C.; Yañez-Falcón, A.R.; Jaimes-Miranda, F.; Garay, E.; Torres-Quiroz, F.; DeLuna, A.; Funes, S. ab’-NAC cooperates with Sam37 to mediate early stages of mitochondrial protein import. FEBS J. 2017, 284, 814–830.
- Lee, D.W.; Hwang, I. Evolution and design principles of the diverse chloroplast transit peptides. Mol. Cells 2018, 41, 161–167.
- Park, M.H.; Zhong, R.; Lamppa, G. Chloroplast stromal processing peptidase activity is modulated by transit peptide determinants that include inhibitory roles for its N-terminal domain and initial Met. Biochem. Biophys. Res. Commun. 2018, 503, 3149–3154.
- Bölter, B. En route into chloroplasts: Preproteins’ way home. Photosyn. Res. 2018, 138, 263–275.
- Day, P.M.; Theg, S.M. Evolution of protein transport to the chloroplast envelope membranes. Photosyn. Res. 2018, 138, 315–326.
- Chotewutmontri, P.; Bruce, B.D. Non-native, N-terminal Hsp70 molecular motor recognition elements in transit peptides support plastid protein translocation. J. Biol. Chem. 2015, 290, 7602–7621.
- Chotewutmontri, P.; Holbrook, K.; Bruce, B.D. Plastid protein targeting: Preprotein recognition and translocation. Int. Rev. Cell Mol. Biol. 2017, 330, 227–294.
- Chang, W.L.; Soll, J.; Bölter, B. The gateway to chloroplast: Re-defining the function of chloroplast receptor proteins. Biol. Chem. 2012, 393, 1263–1277.
- Wiesemann, K.; Simm, S.; Mirus, O.; Ladig, R.; Schleiff, E. Regulation of two GTPases Toc159 and Toc34 in the translocon of the outer envelope of chloroplasts. Biochim. Biophys. Acta 2019, 1867, 627–636.
- Ganesan, I.; Theg, S.M. Structural considerations of folded protein import through the chloroplast TOC/TIC translocons. FEBS Lett. 2019, 593, 565–572.
- Chen, Y.L.; Chen, L.J.; Chu, C.C.; Huang, P.K.; Wen, J.R.; Li, H.M. TIC236 links the outer and inner membrane translocons of the chloroplast. Nature 2018, 564, 125–129.
- Rudolf, M.; Machettira, A.B.; Gross, L.E.; Weber, R.L.; Bolte, K.; Bionda, T.; Sommer, M.S.; Maier, U.G.; Weber, A.P.M.; Shleiff, E.; et al. In vivo function of Tic22, a protein import component of the intermembrane space of chloroplasts. Mol. Plant 2013, 6, 817–829.
- Kasmati, A.R.; Topel, M.; Patel, R.; Murtaza, G.; Jarvis, P. Molecular and genetic analyses of Tic20 homologues in Arabidopsis thaliana chloroplasts. Plant J. 2011, 66, 877–889.
- Flores-Perez, U.; Jarvis, P. Molecular chaperone involvement in chloroplast protein import. Biochim. Biophys. Acta 2013, 1833, 332–340.
- Uniacke, J.; Zerges, W. Chloroplast protein targeting involves localized translation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2009, 106, 1439–1444.
- Sun, Y.; Bakhtiari, S.; Valente-Paterno, M.; Wu, Y.; Law, C.; Dai, D.; Dhaliwal, J.; Bui, K.H.; Zerges, W. Chloroplast-localized translation for protein targeting in Chlamydomonas reinhardtii. bioRxiv 2021.
- Villarejo, A.; Buren, S.; Larsson, S.; Dejardin, A.; Monne, M.; Rudhe, C.; Karlsson, J.; Jansson, S.; Lerouge, P.; Rolland, N.; et al. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 2005, 7, 1224–1231.
- Buren, S.; Ortega-Villasante, C.; Blanco-Rivero, A.; Martinez-Bernardini, A.; Shutova, T.; Shevela, D.; Messinger, J.; Bako, L.; Villarejo, A.; Samuelsson, G. Importance of post-translational modifications for functionality of a chloroplast-localized carbonic anhydrase (CAH1) in Arabidopsis thaliana. PLoS ONE 2011, 6, e21021.
- Bhushan, S.; Kuhn, C.; Berglund, A.K.; Roth, C.; Glaser, E. The role of the N-terminal domain of chloroplast targeting peptides in organellar protein import and miss-sorting. FEBS Lett. 2006, 580, 3966–3972.
- Lee, D.W.; Lee, S.; Lee, J.; Woo, S.; Razzak, M.A.; Vitale, A.; Hwang, I. Molecular mechanism of the specificity of protein import into chloroplasts and mitochondria in plant cells. Mol. Plant 2019, 12, 951–966.
- Lee, D.W.; Hwang, I. Understanding the evolution of endosymbiotic organelles based on the targeting sequences of organellar proteins. New Phytol. 2021, 230, 924–930.
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441.
- Garg, S.G.; Gould, S.B. The role of charge in protein targeting evolution. Trends Cell Biol. 2016, 26, 894–905.
- Sharma, M.; Bennewitz, B.; Klösgen, R.B. Rather rule than exception? How to evaluate the relevance of dual protein targeting to mitochondria and chloroplasts. Photosynth. Res. 2018, 138, 335–343.
- McKinnon, L.; Theg, S.M. Determinants of the specificity of protein targeting to chloroplasts or mitochondria. Mol. Plant 2019, 12, 893–895.
- Bruce, B.D. Chloroplast transit peptides: Structure, function and evolution. Trends Cell Biol. 2000, 10, 440–447.
- Daras, G.; Rigas, S.; Tsitsekian, D.; Zur, H.; Tuller, T.; Hatzopoulos, P. Alternative transcription initiation and the AUG context configuration control dual-organellar targeting and functional competence of Arabidopsis Lon1 protease. Mol. Plant 2014, 7, 989–1005.
- Ye, W.; Spanning, E.; Glaser, E.; Maler, L. Interaction of the dual targeting peptide of Thr-tRNA synthetase with the chloroplastic receptor Toc34 in Arabidopsis thaliana. FEBS Open Biol. 2015, 5, 405–412.
- Lee, D.W.; Lee, S.; Min, C.K.; Park, C.; Kim, J.M.; Hwang, C.S.; Park, S.K.; Cho, N.-H.; Hwang, I. Cross-species functional conservation and possible origin of the N-terminal specificity domain of mitochondrial presequences. Front. Plant Sci. 2020, 11, 64.
- Garrido, C.; Caspari, O.D.; Choquet, Y.; Wollman, F.A.; Lafontaine, I. Evidence supporting an antimicrobial origin of targeting peptides to endosymbiotic organelles. Cells 2020, 9, 1795.
- Caspari, O.D.; Lafontaine, I. The role of antimicrobial peptides in the evolution of endosymbiotic protein import. PLoS Pathog. 2021, 17, e1009466.
- Rascón-Cruz, Q.; González-Barriga, C.D.; Iglesias-Figueroa, B.F.; Trejo-Muñoz, J.C.; Siqueiros-Cendón, T.; Sinagawa-García, S.R.; Alevaro-Gallegos, S.; Espinoza-Sánchez, E.A. Plastid transformation: Advances and challenges for its implementation in agricultural crops. Electron. J. Biotechnol. 2021, 51, 95–109.
- Rozov, S.M.; Sidorchuk, Y.V.; Deineko, E.V. Transplastomic plants: Problems of production and their solution. Russ. J. Plant Physiol. 2022, 69, 20.
- Nishimura, K.; Kato, Y.; Sakamoto, W. Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol. 2016, 171, 2280–2293.
- Rybicki, E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637.
- Sticklen, M. Plant genetic engineering to improve biomass characteristics for biofuels. Curr. opinion biotechnol. 2006, 17, 315–319.
- Hyunjong, B.; Lee, D.-S.; Hwang, I. Dual targeting of xylanase to chloroplasts and peroxisomes as a means to increase protein accumulation in plant cells. J. Exp. Bot. 2005, 57, 161–169.
- Kavanagh, T.A.; Jefferson, R.A.; Bevan, M.W. Targeting a foreign protein to chloroplasts using fusions to the transit peptide of a chlorophyll a/b protein. Mol. Gen. Genet. 1988, 215, 38–45.
- Köhler, R.H.; Cao, J.; Zipfel, W.R.; Webb, W.W.; Hanson, M.R. Exchange of protein molecules through connections between higher plant plastids. Science 1997, 276, 2039–2042.
- Panstruga, R.; Hippe-Sanwald, S.; Lee, Y.-K.; Lataster, M.; Lipka, V.; Fischer, R.; Liao, Y.C.; Häusler, R.E.; Kreuzaler, F.; Hirsch, H.-J. Expression and chloroplast-targeting of active phosphoenolpyruvate synthetase from Escherichia coli in Solanum tuberosum. Plant Sci. 1997, 127, 191–205.
- Jang, I.-C.; Nahm, B.H.; Kim, J.-K. Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol. Breed. 1999, 5, 453–461.
- Kim, E.H.; Suh, S.C.; Park, B.S.; Shin, K.S.; Kweon, S.J.; Han, E.J.; Park, S.H.; Kim, Y.S.; Kim, J.K. Chloroplast-targeted expression of synthetic cry1Ac in transgenic rice as an alternative strategy for increased pest protection. Planta 2009, 230, 397–405.
- Kim, S.; Lee, D.-S.; Choi, I.S.; Ahn, S.-J.; Kim, Y.-H.; Bae, H.-J. Arabidopsis thaliana Rubisco small subunit transit peptide increases the accumulation of Thermotoga maritima endoglucanase Cel5A in chloroplasts of transgenic tobacco plants. Transgenic Res. 2010, 19, 489–497.
- Meyers, A.; Chakauya, E.; Shephard, E.; Tanzer, F.L.; Maclean, J.; Lynch, A.; Williamson, A.-L.; Rybicki, E.P. Expression of HIV-1 antigens in plants as potential subunit vaccines. BMC Biotechnol. 2008, 8, 53.
- Maclean, J.; Koekemoer, M.; Olivier, A.J.; Stewart, D.; Hitzeroth, I.I.; Rademacher, T.; Fischer, R.; Williamson, A.L.; Rybicki, E.P. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: Comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J. Gen. Virol. 2007, 88, 1460–1469.
- Zahin, M.; Joh, J.; Khanal, S.; Husk, A.; Mason, H.; Warzecha, H.; Ghim, S.J.; Miller, D.M.; Matoba, N.; Jenson, A.B. Scalable production of HPV16 L1 protein and VLPs from tobacco leaves. PLoS ONE 2016, 11, e0160995.
- Yanez, R.J.R.; Lamprecht, R.; Granadillo, M.; Torrens, I.; Arcalis, E.; Stoger, E.; Rybicki, E.P.; Hitzeroth, I.I. LALF32-51-E7, a HPV-16 therapeutic vaccine candidate, forms protein body-like structures when expressed in Nicotiana benthamiana leaves. Plant Biotechnol. J. 2018, 16, 628–637.
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-targeted, nanoparticle-based drug-delivery systems: Therapeutics for mitochondrial disorders. Life 2022, 12, 657.
- Palmer, C.S.; Anderson, A.J.; Stojanovski, D. Mitochondrial protein import dysfunction: Mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 2021, 595, 1107–1131.
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597.
- Lopez-Torrejon, G.; Jimenez-Vicente, E.; Buesa, J.M.; Hernandez, J.A.; Verma, H.K.; Rubio, L.M. Expression of a functional oxygen-labile nitrogenase component in the mitochondrial matrix of aerobically grown yeast. Nat. Commun. 2016, 7, 11426.
- Baysal, C.; Pérez-González, A.; Eseverri, Á.; Jiang, X.; Medina, V.; Caro, E.; Rubio, L.; Christou, P.; Zhu, C. Recognition motifs rather than phylogenetic origin influence the ability of targeting peptides to import nuclear-encoded recombinant proteins into rice mitochondria. Transgenic Res. 2020, 29, 37–52.
- Jiang, X.; Coroian, D.; Barahona, E.; Echavarri-Erasun, C.; Castellanos-Rueda, R.; Eseverri, Á.; Aznar-Moreno, J.A.; Buren, S.; Rubio, L.M. Functional nitrogenase cofactor maturase NifB in mitochondria and chloroplasts of Nicotiana benthamiana. mBio 2022, 13, e00268-22.
