Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Polyvinylidene fluoride (PVDF), the chemical formula is (C2H2F2)n. Its basic building blocks are therefore carbon, hydrogen, and fluorine. These three elements can form several crystalline chain conformations. Conformations are defined by polar and nonpolar phases. Four phases are most commonly found in the literature: α-, β-, γ-, and δ-.
- degradation
- electrospinning
- fabrication
- nanofibers
1. Introduction
Nowadays, there are countless synthetically produced polymers with different properties and functions. Organic polyvinylidene fluoride (PVDF) can be classified as one of the most interesting. The reason that has made it so popular is due to its vast range of applications precisely because of its properties [1]. This semicrystalline fluoropolymer has a considerable chemical resistance, high mechanical strength, extensive operating temperature range (the glass transition temperature is −35 °C and the melting point is 177 °C). Moreover, it is biocompatible and highly hydrophobic. It can be argued that similar properties can be achieved with other types of polymers, which is certainly true. For example, polytetrafluoroethylene (PTFE) is regarded as an ideal alternative, but quite expensive [2]. As another similar, fluorinated ethylene propylene (FEP) can be considered, which is suitable for generators in an outdoor environment [3,4,5]. However, PVDF has one other unique property, and that is the ability to generate a charge, not only using the triboelectric effect but also using piezoelectricity, which is a great advantage over other polymers. Thus, PVDF can rightly be called a nanogenerator. Scientists in many fields are exploring the combination of all these properties and the ability to generate a charge [6,7,8,9,10,11]. This paper selects a few of the most widely used and interesting ones. In general, these energy harvesters have taken the name of piezoelectric nanogenerators (PENGs) or triboelectric nanogenerators (TENGs). Although there are many other and not less interesting representatives of both PENGs or TENGs [12], PVDF can also act as a hybrid in both cases [10], and this capability makes it quite distinctive.
First of all, it is necessary to describe PVDF in terms of its chemical structure. The chemical formula is (C2H2F2)n. Its basic building blocks are therefore carbon, hydrogen, and fluorine. These three elements can form several crystalline chain conformations [13]. Conformations are defined by polar and nonpolar phases. Four phases are most commonly found in the literature: α-, β-, γ-, and δ-. Less commonly mentioned is a fifth ϵ-phase [14]. Phases α and ϵ belong to the nonpolar ones. Molecules with antiparallel packing of the dipoles are nonpolar bonds and have no dipole moment [14]. Conversely, polar molecules do not have a full covalent bond, so an imbalance in the electron charge of the molecule is present. The imbalance in the distribution of electrons generates dipoles. The dipoles will try to align themselves when an electric field is provided. The polarity of a molecule affects the attraction between molecular chains. Furthermore, nonpolar polymers are less permeable to water than polar polymers [15]. Thus, it is clear that the polar phases are the most interesting to observe in the case of charge generation. The most associated with charge generation is the β-phase [14]. Furthermore, not neglected is the polar γ-phase, where its polarization effect is weaker. This is because the gauche bond exists in every fourth repeat unit [16]. The often mentioned nonpolar α-phase is usually obtained from the melt by crystallization. The phase conformations of PVDF polymer are illustrated in Figure 1, where their different structures can be clearly seen.
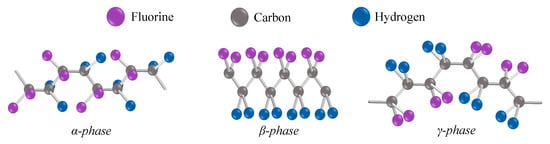
Figure 1. The chain conformation of the most observed phases in PVDF [17]. Because the fluorine atoms in the β-phase are situated on the same side of the molecular chains, which are arranged parallel to one another in a specific direction, with the same dipole orientation and enhanced polarity, the β-phase exhibits spontaneous polarization strength as well as pyro- and piezoelectric properties [18].
Popular methods for phase characteristics are Fourier transform infrared spectroscopy (FTIR), Raman Spectroscopy, X-ray diffraction (XRD), and differential scanning calorimetry (DSC).
2. Material Reactions and Degradation
As already mentioned, PVDF is a nonreactive polymer and has a high toxic resistance. Its resistance to degradation can be considered higher compared to other polymers [161]. Changes occur mainly with higher temperatures and standardly may be combinations of two or more effects (mechanical properties, crystallinity, color, etc.) [162]. It is commonly able to resist basic solutions, chlorine solution, alcohols, several acids, halogens, and aliphatic or aromatic compounds [19]. PVDF weakens when various alkaline solutions are used [163,164]. Lactic acid C3H6O3, nitric acid HNO3, sulfuric acid H2SO4, and tetrahydrofuran C4H8O are mentioned as having little resistance to acids. Its color may change from pure white to yellow, and during the dehydrochlorination process (loss of hydro fluoride units from the polymer chain), it may darken to black [165]. Furthermore, less resistant are glucose and wine vinegar, which is essentially a concentrate of acetic acid. When exposed to higher temperatures, the damage caused by these substances increases.
Regarding thermal degradation, temperature can affect charge generation mainly because of the weakening of mechanical properties at very low temperatures, i.e., the glass transition temperature of −35 °C, when the material hardens and becomes more brittle. Below this critical value, the material may degenerate as it loses its elasticity and flexibility, which naturally limits the development of piezoelectric phenomena. This temperature is relatively low compared to commonly known polymers. For example, PTFE has Tg = 115 °C. On the other hand, at the melting temperature of 177 °C, the crystallization process is affected. For PTFE, Tm = 327 °C. Exceeding any of these threshold temperatures can lead to a different phase transition of the material, which is addressed, for example, by the previously mentioned DSC [166]. Despite all the mentioned drawbacks, PVDF is very stable within these values and it is often called a thermoplastic polymer. The temperatures mentioned may vary slightly in units of degrees depending on the manufacturer of the commercial polymer type.
For PVDF, which has flexible fibers, the critical issue is mechanical strength which is, of course, very different from solid layers. In fact, a good tensile strength is required for a piezoelectric generator. If the nanofibers do not have additional support, they can be damaged more quickly. Microcracks and point defects can already occur with imperfect fabrication, which can be, for example, a contamination of the microparticles. However, it can still be argued that cracks may not be spread throughout the material in the case of single fibers as opposed to solid layers. The tensile strength, elongation at break, and Young’s modulus of the PVDF fiber were measured by Hasim, Liu, and Li [167] in sodium hydroxide NaOH solutions. The results in degradation changes were classified as significant. Here again, the increased temperature also accelerated the aging of the samples.
3. Utilization in Real Applications
Although PVDF can be used as a nanogenerator of energy, it is a relatively broad concept. Therefore, it is appropriate to mention several interesting works that are experimentally devoted to specific applications. Hence, this section does not primarily serve to describe the various fillers and other composite combinations that can improve PVDF properties, but to describe the direct applications of PVDF itself.
A widespread and intended use of PVDF as a nanofabric is directly for the human body. There can be several uses. The simplest uses can be as a wearable shirt, generating charge when walking and moving [168], during inhaling and exhaling [169], or blood flow [170,171,172], or as gloves [173]. It can also serve as a shoe insole [49,174,175,176], or while typing on the keyboard [177]. For these applications, PVDF can operate just as a common sensor (safety monitoring, medical diagnostics), or as a nanogenerator depending on its performance [40,178]. In these cases, the connection to the IoT is also assumed [179]. In addition to such practical uses, PVDF can also be used purely as a green energy harvester, for example, for wind energy harvesting [3,5,180], or for energy harvesting from ocean waves and applications [181,182].
Among the less common but emerging uses may include the use of PVDF as scaffolds in tissue engineering due to its biocompatibility. For example, for osteoblasts (bone cells), where their electromechanical stimulation can accelerate cell spreading [183,184,185]. For solar cells, PVDF can serve as an enhancer of the crystallinity of currently very popular perovskites [186], when there are already attempts to create a hybrid inducing the piezophototronic effect [187]. Another unique use of the PVDF nanogenerator also appears to be energy harvesting from sound waves [188,189].
This entry is adapted from the peer-reviewed paper 10.3390/coatings12101429
This entry is offline, you can click here to edit this entry!
