Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
COVID-19, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), mainly attacks cells in the respiratory system. In the acute phase of COVID-19, patients with moderate–to–severe ARDS are characterized by an elevated pro-inflammatory state secondary to a “cytokine storm” (CS). This process stimulates the generation of reactive chemical species (RS) and induces oxidative stress (OS), that has been postulated as the primary cause of tissue damage and consequent functional impairments post-COVID-19.
- post-COVID-19
- exercise
- physical activity
- rehabilitation
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has significantly increased the number of patients hospitalized for pneumonia and acute respiratory distress syndrome (ARDS) [1]. COVID-19, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), mainly attacks cells in the respiratory system [2,3]. In the acute phase of COVID-19, patients with moderate–to–severe ARDS are characterized by an elevated pro-inflammatory state secondary to a “cytokine storm” (CS) [4,5,6,7]. This process stimulates the generation of reactive chemical species (RS) and induces oxidative stress (OS) [8,9,10], that has been postulated as the primary cause of tissue damage and consequent functional impairments post-COVID-19 [10,11,12]. Fatigue and muscle weakness are the primary impairments reported in patients post-COVID-19 even six months after medical discharge [13]. These prolonged sequalae underscores the longstanding impact of the heightened inflammatory process and OS, muscle damage and sarcopenia secondary to viral infection, and the prolonged hospital stay.
Exercise is the cornerstone of pulmonary rehabilitation (PR) programs that is essential to restore self–autonomy and the ability to perform meaningful activities of daily living in these patients (including sessions of strength and endurance or aerobic exercises). Regarding aerobic exercise, the type and intensity of training should be adequately prescribed by the health professional to be well tolerated by patients and achieve the high adherence required to reverse the physical impairments. The prescribed exercise needs to promote muscle’s function and mass while minimizing cardiopulmonary stress. The mainstay exercise in PR is concentric (CONC) in nature, which unfortunately stimulates significant cardiopulmonary stress to reach the exercise intensities that induce clinical improvements. In contrast, eccentric (ECC) exercises, when muscles contract while lengthening, improve muscle function and mass with minimum cardiopulmonary stress; this is a relative novel mode of clinical training despite its high requirement during daily activities (e.g., walking downstairs or hills, sitting down in a chair). Substantial evidence indicates that ECC compared to CONC training induces improvement in functional capacity with less dyspnoea and fatigue [14,15,16]. Whether these benefits occur in patients with moderate–to–severe damage by COVID-19 is not known.
2. Role of Inflammatory and OS Markers in COVID-19
The cellular entry of SARS-CoV-2 depends on the viral structural spike protein binding to angiotensin-converting enzyme 2 (ACE2) receptor, and the fusion of membranes mediated by the type 2 transmembrane serine protease (TMPRSS2) of the host cells [2,35]. The ACE2 receptor and TMPRSS2 are expressed mainly in the respiratory system, but also by the intestine, kidneys, and heart [36,37]. The lungs are the main target organ of SARS-CoV-2, due to their large surface area exposed inhaled infectious agents and the nature of type II epithelial cells [38]. Inside the cell, SARS-CoV-2 releases their RNA, and more virions are able to replicate. When the virus is recognized, macrophages and neutrophils are recruited to the infection site, initiating an overproduction of cytokines and consequent CS [6,7]. Moreover, interleukin–1β (IL–1β), IL–6, IL–10, interferon-gamma (IFN–γ), and tumor necrosis factor-alpha (TNF–α) are the major inflammatory markers elevated in patients infected by SARS-CoV-2 contributing to the CS [39,40,41,42,43].
Cytokines are proteins that act as signaling molecules that recruit immune cells to the site of inflammation, induce vascular leakage and exudation, and stimulate the formation of reactive species (RS) to eliminate the virus, promoting OS. The OS is defined as an imbalance between RS and antioxidants in favor of the RS, leading to a disruption of redox signaling and control, and/or molecular damage [44]. The evidence suggests that the overproduction of RS and/or a low quantity of antioxidants is associated with the pathogenesis, progression, severity, and sequelae induced by SARS-CoV-2 infection [45,46]. To understand the origin of the dysfunctions seen in patients with COVID-19, it is necessary to recognize the role of RS as the factors executing tissue damage and organ deterioration.
3. Redox Imbalance in Patients with COVID-19
The RS are produced by reactions during respiration and can be generated by phagocytic cells including neutrophils and macrophages [47]. An overview of the main RS is shown in Figure 1.
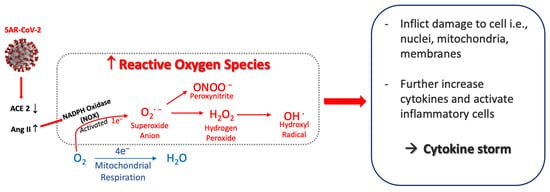
Figure 1. Postulated sequence that generates reactive oxygen species from SARS-CoV-2. SARS-CoV-2 infection induces a decrease in ACE2 that causes Ang II to increase, which activates NADPH oxidase (NOX). This generates superoxide anion (O2·−) and other oxygen radicals—peroxynitrite, hydrogen peroxide and hydroxyl radical. The reactive oxygen species inflict cellular damage, further activate inflammatory cells, and amplify the increased release of cytokines. Abbreviations: ACE2, angiotensin–converting enzyme 2; Ang II, angiotensin II.
In viral infections, a major main source of RS production is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), the main enzymes expressed by macrophages [48]. In COVID-19, NOXs have been recognized as the main source of RS formation. The binding of SARS-CoV-2 to the ACE2 receptor results in increases in angiotensin II (Ang II) because the ACE2 receptor is no longer available to convert Ang II to Ang 1–7 (renin-angiotensin-aldosterone system, RAAS). Subsequently, Ang II binds the angiotensin type 1 receptor (AT1R) and stimulates NOXs activity. NOXs act by reducing O2 to superoxide anion (O2−) [49] leading to overproduction of O2− [50], which in turn can initiate production of other oxygen radicals.
The O2− can react with other reactive species to produce hydrogen peroxide (H2O2) [51]. H2O2 is relatively stable with a prolonged half-life so often measured at systemic or respiratory levels as marker of RS. Nonetheless, it is considered cytotoxic at high concentrations because it can generate the very harmful RS, the hydroxyl radical (OH−). OH− can damage inorganic and organic molecules, including lipids, proteins, carbohydrates, and DNA. It has a short half–life, and its high reactivity allows it to damage molecules very close to its formation site. Due to its reactivity, it is impossible to determine its quantity or concentration, so it is common to evaluate the damage associated with their production [52], such as derivates of arachidonic acids oxidation and prostaglandins (e.g., 8–isoprostane, 8–iso–PGF2α) [53].
Another source of RS production implicated in viral infections is inducible nitric oxide synthase (iNOS) enzyme present in macrophages and neutrophils [54,55]. iNOS rapidly produces high levels of nitric oxide (NO·), and their derivates nitrite (NO2−) and nitrate (NO3−) for reducing pathogens [56]. The NO· in the presence of O2− from NOXs reactions can produce peroxynitrite (ONNO−), a highly harmful RS that generates lipid peroxidation of biological membranes and 8–iso–PGF2α formation [53]. Add to this RS increases, in viral infections have been found the inhibition of pathways mediated by the nuclear factor erythroid related factor 2 (Nrf2), which is a master transcription regulator of genes related to antioxidant enzymes necessaries to counteract the increases of O2− and H2O2 (superoxide dismutases (SODs), glutathione peroxidases (GPXs), and catalase (CAT)) [57,58]. Concerning, have been reported a direct association between low expression of SOD and disease severity in lungs of elderly patients with COVID-19 [59].
It is considered that OS in at risk COVID-19 patients is due to an excess of RS that is not countered by an increase in antioxidants. OS levels in these patients have been determined from an increase in inflammatory markers such as cytokines. However, this evidence is mainly supported by review articles. To our knowledge, only one study has evaluated in patients with COVID-19 inflammatory and OS markers (H2O2, malondialdehyde (MDA), and total antioxidant capacity (TAC)) by blood samples, finding no correlation with the severity of the disease [60]. Therefore, investigations are needed to document inflammatory and OS markers in COVID-19 patients to evaluate their association with tissue damage and consequent functional impairments in the acute phase of the disease. Future studies need to not only evaluate measures of their systemic effect (blood or urine samples) but also markers of the respiratory system (e.g., exhaled breath condensate (EBC) samples); the latter of which is essential to understand the primary target organ of SARS-CoV-2 and its greater susceptibility to tissue damage.
4. Effect of Aerobic Exercise/Training on Inflammatory and OS Markers
It is well described that acute exercise induces an increase in pro–inflammatory cytokines and OS markers [79,80,81], being more pronounced after bouts of ECC than CONC exercises [82,83]. Of interest, chronic CONC and ECC exercise not only leads to a reduction in these outcomes [84,85,86,87], but patients also benefit by increases in functional capacity, HRQoL, reduction in hospitalization, morbidity, and mortality. To date, there are not similar data from patients post-COVID-19 or recovered from others coronavirus infections (i.e., SARS-CoV-1 and MERS). Thus, evidence is lacking regarding the impact of the virus infection on biological response to physiological stress induced by exercise.
A commonly used protocol to evaluate the training-induced changes utilizes serial analyses of biological samples before and after the completion of an acute submaximal exercise; thus, to evaluate the adaptations induced by training, the changes of markers during the protocol are analyzed. This procedure mitigates interpretation errors associated with the intrinsic variability of markers commonly used in clinical studies. By this method, Mercken et al. [88] reported that 8-weeks of CONC exercise in 11 patients with moderate COPD decreased the hydrogen peroxide (H2O2) in EBC samples and malondialdehyde (MDA) in blood and increased the exercise capacity (VO2-peak and 6mWT). Similar results were reported by Rodriguez et al. [89] in 18 patients with severe COPD. Based on these results, it can be deduced that training decreases OS markers and increases functional capacity in respiratory diseases; however, it is not known if the effect differs depending on the training mode performed (ECC vs. CONC). The analysis of these markers can better inform how disease phenotype or respiratory conditions, with or without comorbidities, respond to different types of training.
Considering previous studies, ECC training has the potential to be a valuable alternative for patients with limited capacity to reach and sustain sufficient CONC exercise intensities to induce significant functional gains. To our knowledge, the physiologic response and benefit of ECC exercises in post-COVID-19 patients is unexplored. Thus, it is necessary to evaluate its clinical impact by comparing supervised ECC versus CONC training on functional capacity and related variables in outpatients with moderate-to-severe damage by COVID-19 to identify the most well tolerated and effective training method to apply in PR programs.
5. Aerobic Eccentric Training for Pulmonary Rehabilitation in Patients Post-COVID-19
Patients post-COVID-19 have reduced lower limb muscle mass and strength [90], specifically the knee extensors muscles (i.e., quadriceps muscle). This impairs functional capacity and self–autonomy, resulting in sedentary behaviors and exacerbating leg fatigue symptoms, muscle weakness during daily activities, and sarcopenia [91,92]. Thus, the training mode performed during PR programs should be well tolerated by patients and effective for improving their functional capacity, autonomy and HRQoL. The conventional aerobic exercises included in the standard of PR are cyclic movements, mainly focused on lower limbs (e.g., running, jogging, or walking (depending of the functional impairments), actions that implicate CONC and ECC contractions, but being the CONC the more predominantly where the treadmill is set up with positive slope) and cycling (with CONC as the primary movements)).
To effectively improve skeletal muscle performance, exercise training load must be greater than the regular daily physical activity [93]. Although CONC are commonly used and relatively safe, it induces a significant cardiovascular and respiratory stress when exercise is completed at moderate-to-severe intensity. Dyspnea followed by leg fatigue are primary patient reported outcomes for stopping exercise in chronic cardiorespiratory diseases [94,95,96]. In post-COVID-19 patients, it has been proposed that hypermetabolism, secondary to an increased catabolic process induced by SARS-CoV-2 infection [97], could further impede their ability to reach and sustain sufficiently high exercise intensities to promote the beneficial training–induced changes.
ECC contractions, when muscle lengthens while producing force, it is a relatively novel mode of training proposed to be potentially effective for patients with exercise intolerance [98,99,100,101]. ECC training can induce greater gains in muscle power while inducing lower metabolic and cardiorespiratory demands than CONC training (by 4 to 5 fold) [98,101,102]. ECC could be implemented during cycling or treadmill walking, using special ergometers. An ECC cycle ergometer utilizes an electrical motor that moves the cranks backwards and consequently is countered by ECC contractions mainly of the knee extensor muscle group [103].
ECC training appears to induce lesser cardiac and ventilatory stress than CONC training. Compared with CONC, the oxygen cost to be considerably lower (about 1/5 of CONC) [104], and, for a given level of VO2 (higher 1 L·min−1) cardiac output is higher and systolic volume lower during ECC training [104,105]. This enables patients to perform greater workloads of muscle power in a session than traditional CONC cycling training under comparable cardiorespiratory stress [106,107]. Regarding ventilatory stress induced by ECC versus CONC training, healthy subjects have demonstrated lower minute ventilation, tidal volume and hyperpnea at the same intensity [108].
Related to a lower cardiorespiratory stress, ECC training has demonstrated benefits in COPD patients. Ward et al. (2021) recently reported that ECC cycling was described to be more enjoyable than CONC by COPD patients. It was also associated with lower production of lactate and creatine kinase levels (markers of glycolysis and muscle damage, respectively) [109]. Moreover, MacMillan et al. (2017) reported that COPD patients were able to perform ECC cycling at a 3-fold higher workload with lower fatigue and dyspnoea during 10 weeks of training when compared to CONC cycling. Further, ECC cycling increased knee extensor strength by 16% and lower limb muscle mass by 2%, with no commensurate changes in the CONC cycling group [106]. Recently, Inostroza et al. (2022) reported that 34 sessions (30 min, 5–6 to RPE) in COPD patients produced a 3–fold greater workload, 1.5% higher SpO2, 24% lower hear rate (HR), 64% lower dyspnoea, and notably improved functional capacity (25% of 6mWT distance) than CONC training [15]. Camilo et al. (2015) reported in COPD patients that walking downhill showed lower minute ventilation and VO2 at a similar exercise intensity than CONC walking (9 and 10% less about peak values, respectively) [110]. Further, they found that 12–weeks of ECC training (−10% decline at treadmill) induced more COPD patients to achieve the 30 m of change at 6MWT than conventional CONC (94% vs. 65%), with a faster progression of treadmill speed and lower dyspnoea [111]. Taken together, ECC cycling, and walking induced considerable physiologic benefits and functional improvements while imposing lower degree of dyspnoea.
This entry is adapted from the peer-reviewed paper 10.3390/biology11101446
This entry is offline, you can click here to edit this entry!
