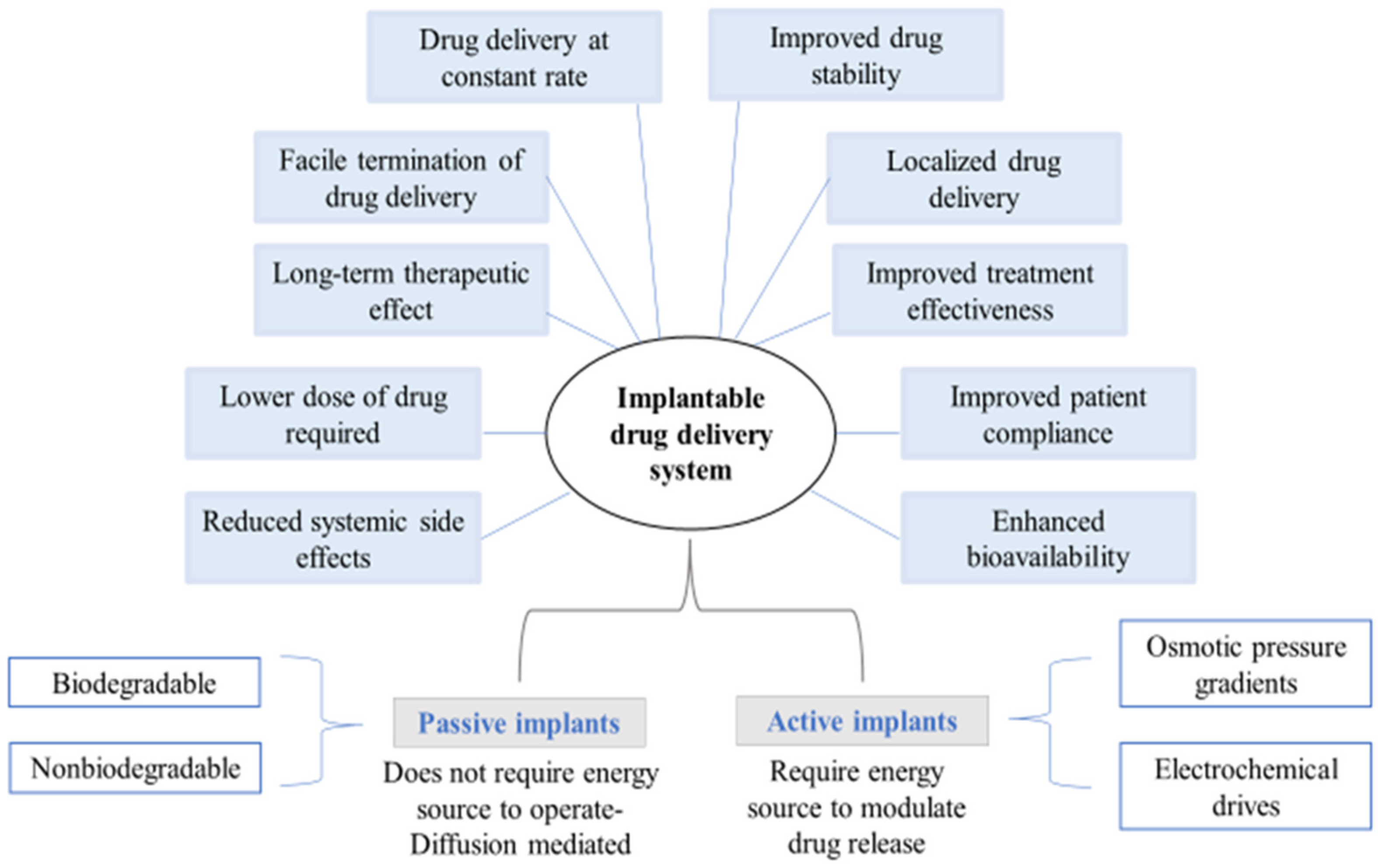
Implantable drug delivery systems advocate a wide array of potential benefits, including effective administration of drugs at lower concentrations and fewer side-effects whilst increasing patient compliance. Amongst several polymers used for fabricating implants, biopolymers such as polysaccharides are known for modulating drug delivery attributes as desired.
- implants
- polysaccharides
- biopolymers
- drug delivery
1. Introduction
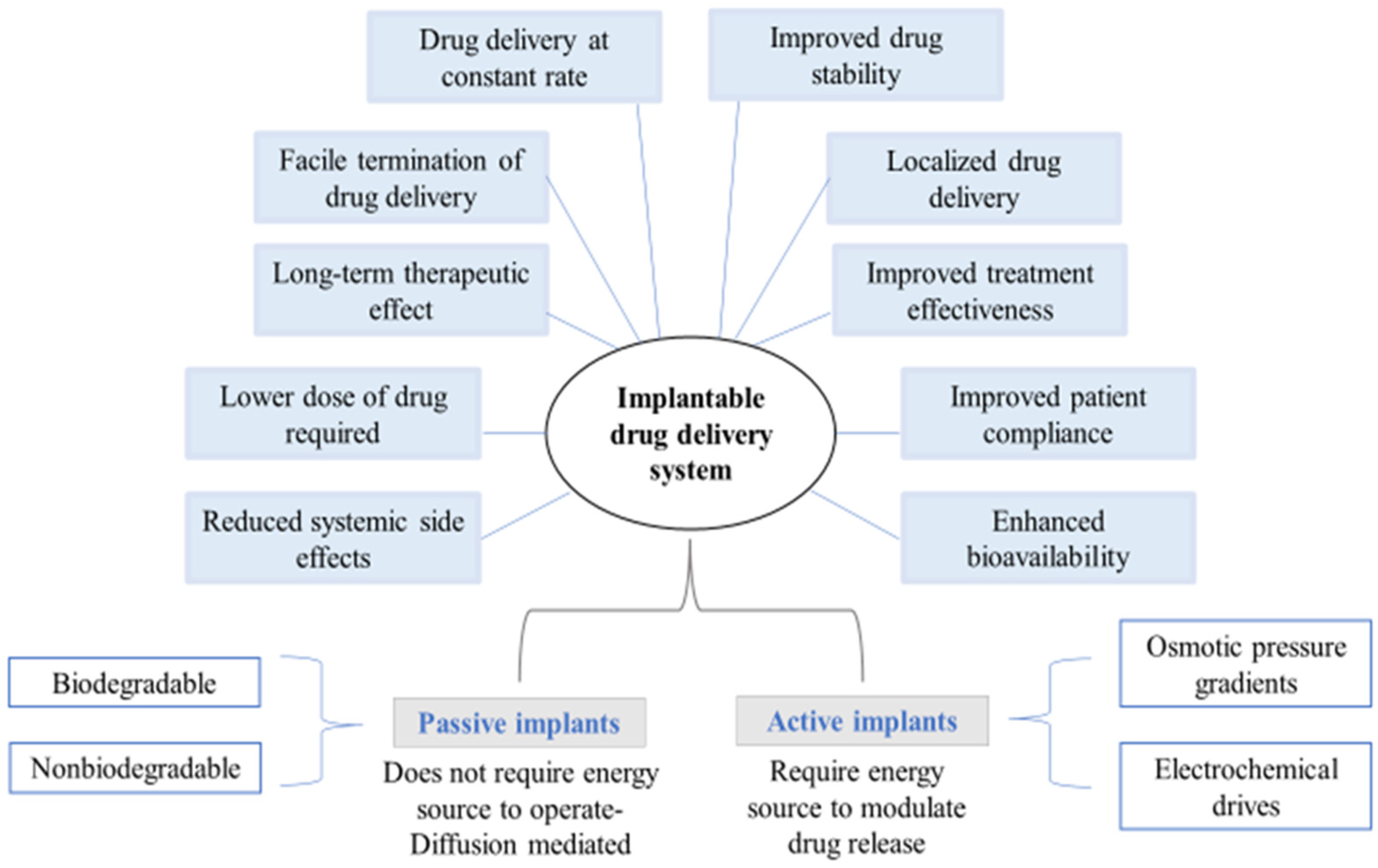
2. Classification of Implantable Drug Delivery Devices
| Origin | Polysaccharides |
|---|---|
| Plant/algal | Starch (amylose/amylopectin), cellulose, agar, alginate, carrageenan, pectin, konjac, guar gum |
| Animal | Chitin/chitosan, hyaluronic acid |
| Bacterial | Xanthan, dextran, gellan, levan, curdlan, polygalactosamine |
| Fungal | Pullulan, elsinan, yeast glucans |
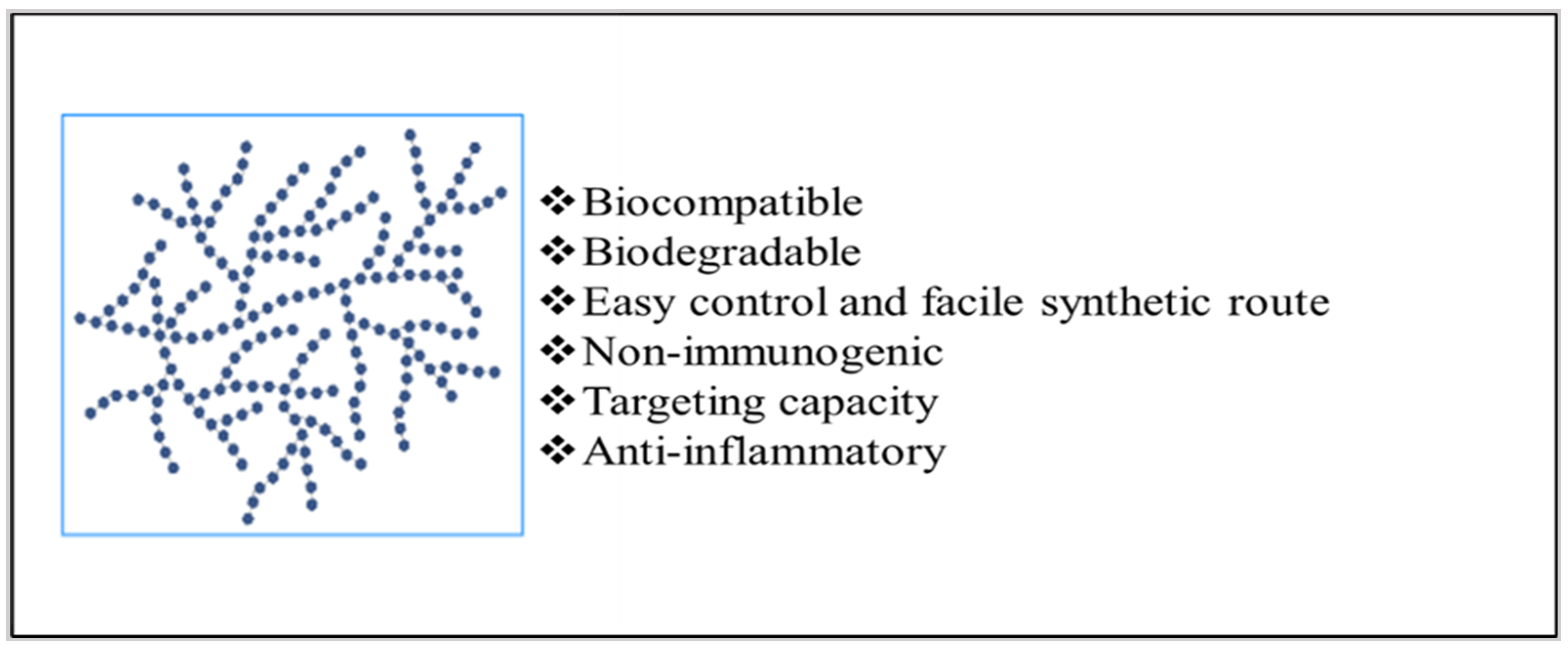
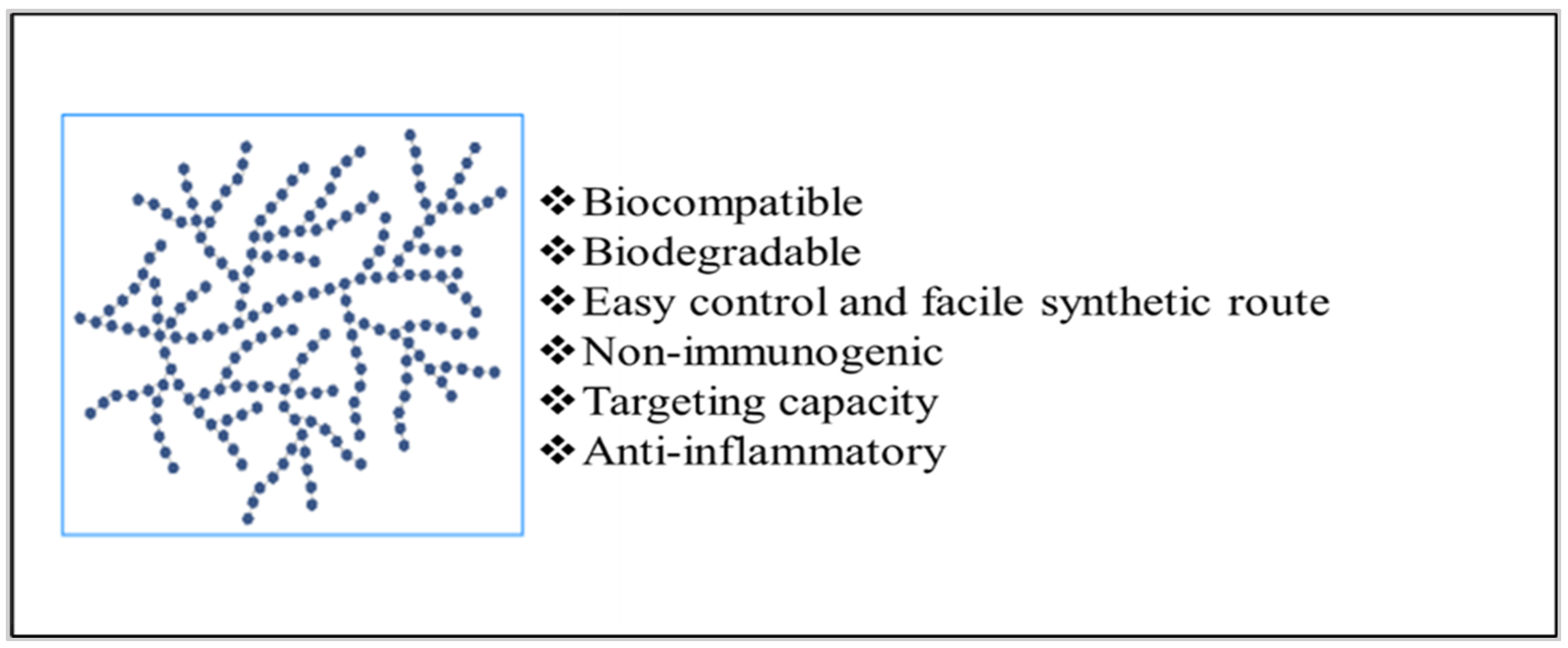
3. Strategies to Employ Polysaccharides in Implant Formulation
Implants can either be entirely made up of polysaccharides, contain polysaccharides as a part of the polymeric blend [7], or be coated with polysaccharides depending upon the therapeutic action required [8][9][10]. Figure 4 displays the representation of these strategies for developing polysaccharide-based implants.
4. Polysaccharide Based Polymers
4.1. Starch
Chemical modification of the surface-active hydroxyl functional groups in starch confers controlled release characteristics to it that aids in the efficient delivery of therapeutic molecules to the target site. The widely used methods of modification include esterification, etherification, oxidation, cross-linking, and cationization, that ultimately lead to alteration in the physicochemical properties of starch. Each glucose unit in starch consists of three free hydroxyl groups that, in the presence of oxidizing agents, under controlled temperature and pH conditions, yield carbonyl or carboxyl derivatives of reduced viscosity, high clarity, improved swellability, and better stability. Oxidation alters the surface morphology of the starch matrix, resulting in controlled biodegradation of the polymer and sustained release of the active agent over a specific period of time [12]. Etherification involves the alkali-catalyzed interaction of starch and alkyl oxides to yield hydroxyalkyl starches that promote disruption of internal bonds and enhance the freedom of motion in the amorphous region of starch granules. The weakening of bonds between starch chains depends on the degree of hydroxypropyl group substitution, which in turn enhances the solubility, ease of hydration, swelling power, and enzyme digestibility. On subjection to freeze-thaw cycling, hydroxypropyl groups, being hydrophilic in nature, prevent the separation of water through syneresis, thus enhancing the freeze-thaw stability [13]. Acetylated starch is an example of ester modification, where the substitution by acyl groups confers hydrophobicity to the starch molecules and enhances their thermoplastic character. Hydrogen bond disorientation induced by acetylation in native starch, results in retardation of crystallization and a lowering of pasting temperature that in turn enhances its swelling power [14]. The introduction of ammonium, phosphonium, imino, or sulfonium groups confers a positive ionic charge on starche and this process is referred to as cationization. The modification method can be either dry or wet. Dry cationization involves spraying the cationic molecules on dried starch in the absence of a liquid medium, whereas wet cationization involves a liquid medium mediated reaction between starch and the cationic molecules. The characteristic properties exhibited by cationic starches include improved dispersibility, solubility, and stability [15]. Cross-linking of starch is facilitated by the use of polyfunctional reagents such as sodium trimetaphosphate, phosphorus oxychloride, genipin, and epichlorohydrin that form covalent bonds with the starch granules, making the ordering of the internal granules denser than its native counterparts. Cross-linking improves the tolerance of starch towards processes involving high shear and extreme pH [16].
4.2. Cellulose
Owing to its mechanical strength and biocompatibility, it is widely used in tissue engineering. Even though both plant-based as well as bacteria-based cellulose are natural, considerable differences have been observed between them with respect to purity and macromolecular characteristics. In comparison to plant-based cellulose, bacterial cellulose has a high value of Young’s modulus [17][18][19], a high-water absorption capacity, and a high aspect ratio in its fibers [20]. Cellulose degradability can be induced by oxidation, which is a very effective approach. Many different oxidizing agents, including NaClO2, CCl4, nitrogen oxides, and free nitroxyl radicals, can be used to create oxidized cellulose [21][22]. The remarkable properties such as high absorbability, antiviral and antibacterial effect, non-toxicity, and anti-adhesive qualities have made oxidized cellulose a popular choice for use as a wound healing material [23][24][25].
4.3. Alginate
Extraction of alginate from seaweed is a multistage procedure. First, the dried raw material is treated with dilute mineral acid. Once the alginic acid becomes pure, it is converted into its water-soluble sodium salt in the presence of calcium carbonate, which is then transformed back into acid [26]. Alginate has great potential as a biomaterial for numerous biomedical applications, particularly in drug delivery, wound healing, and tissue engineering. Characteristics like biocompatibility, mild gelling conditions, and simple modifications for preparing alginate derivatives with new properties make it suitable for these possible applications. Alginates undergo acid-mediated hydrolytic cleavage [26]. The reaction consists of three stages: (a) protonation of the oxygen atom at a glycosidic bond; (b) hydrolysis of the conjugate to generate the carboxonium ion and the non-reducing terminus; and (c) fast addition of water molecules onto the carboxonium ion, resulting in the formation of a reducing end. Sodium alginate can be stored as a dry powder at room temperature for several months without undergoing degradation [27].
4.4. Chitosan
The low aqueous solubility and poor acid stability of chitosan limit its use in pharmaceutical products. Various structural modifications are performed at various functional groups for solubility enhancement of chitosan. Modifications at amino, hydroxyl or both amino and hydroxyl group forms N-modified, O-modified or N, O-modified chitosan derivatives. Incorporation of carboxymethyl groups at the C6-hydroxyl group or at the NH2 functional group is an important method for enhancing chitosan’s solubility [28][29].
4.5. Pullulan
Pullulan is a linear, unbranched, neutral exopolysaccharide obtained from the fungus Aureobasidium pullulans that is composed of maltotriose units interconnected by α-D-(1,6) glycosidic bonds. The three glucose units present in each maltotriose unit are in turn connected by α-D-(1,4) glycosidic bonds [30]. The distinctive linkage pattern of pullulan confers unique physicochemical properties to the biopolymer, including oxygen impermeability, adhesiveness, water solubility, and structural flexibility. Based on the strain and fermentation parameters, the molecular weight of pullulan ranges from 45 to 600 KDa, offering polymers of varying viscosities, that play a crucial role in controlling the release of the active agent [31]. Mechanical stability of the pullulan matrix can be enhanced by inter-chain cross linking. In practice, the concentration of crosslinkers should be well optimized to obtain the desirable release as the water absorption capacity depends on the degree of cross-linking. Pullulan is soluble in water, partially soluble in dimethyl formamide and dimethyl sulfoxide, and insoluble in other organic solvents. Imparting hydrophobicity to pullulan by chemical modification is highly desirable for its use in drug delivery applications. Grafting is one such method that involves either the free-radical mediated covalent attachment of hydrophobic monomers onto a polymeric backbone or the incorporation of pre-synthesized grafts to the polymeric chain charged with complementary functional groups [32].
4.6. Carrageenan
4.7. Dextran
Dextran possesses various important properties that make it a useful biopolymer for the development of implantable systems. It is a non-immunogenic, biocompatible, and biodegradable polymer with excellent solubility characteristics. It is generally not degraded when acted upon by the enzymes present in the upper gastrointestinal tract. The activity of dextranase enzymes located in the lumen of the large intestine, liver, kidney, and spleen is responsible for its degradation [38][39]. The molecular weight and branching of dextran dictate its rheological properties. Dextran, having a molecular weight in the range of 40,000 to 2 million, behaves like a Newtonian system up to a concentration of 30% [40]. High molecular weight Dextran shows pseudoplastic behavior. This can be explained by the fact that the forces generated during the application of shear would have broken the structural interaction between α-glucan chains in solution [41].
4.8. Hyaluronic Acid (HA)
HA is a weak polyelectrolyte, and therefore, its rheological properties in aqueous solution are affected by varying conditions of pH, ionic strength, and temperature. The change in pH of the aqueous buffered HA solution significantly affects its stability. HA degradation occurs at acidic (pH < 4) and alkaline (pH > 11) conditions [42]. At very high concentration, the HA solution has a very high viscosity, but on application of pressure, it flows easily. This shear-thinning behavior is due to the breakdown of intermolecular H-bonds and hydrophobic interactions under increasing shear rates [43][44][45].
4.9. Agar
Agarose is a neutral gel-forming molecule. Due to a similarity in their backbone structures, agaropectin and agarose are closely linked. The bacterial species do not enzymatically breakdown agar. Agarose and agaropectin consist of chemical structure alternating between 1,3-linked-B-D-galactopyranose, and 1,4-linked-3,6-anhydro-L-galactopyranose which can be masked to variable degrees by various sugar residues. Agarose is the component with the highest gelling tendency. Agar gels can withstand temperatures of up to 65 °C, but molten agar cannot gel until cooled to roughly 40 °C. Gels made of agar are very transparent [46]. Agar’s exceptional ability to gel is solely due to hydrogen bonds created between its linear galactan chains, which offer high reversibility in gelling and melting points that differ by roughly about 45 °C. Agar was broken down by enzymes and acid to produce agarobiose and neoagarobiose, respectively, which shows that 1, 3-linked-β-D-galactopyranose and 1, 4-linked-3, 6-anhydro-α-L-galactopyranose alternate with agarobiose repeating disaccharide units to make up agarose. Agaropectin has a substantial number of acid groups, such as sulphate, pyruvate, and glucuronate groups, despite having what seems to be the same structural backbone as agarose.
4.10. Pectin
Pectic acids are poly (α-D-galactopyranosyluronic acids) galacturonoglycans that lack or have a very low concentration of methyl ester groups. There are several levels of neutralization for pectic acids. Pectates are the name for the salts of pectic acids [47]. Pectin is a high-value functional food component that is frequently used as a stabilizer and gelling agent. Additionally, it is a plentiful, common, and multipurpose component of all terrestrial plant’s cell walls. Pectin defines a family of oligosaccharides and polysaccharides that share similar properties, yet are exceedingly different in their fine structures [48].
4.11. Gellan Gum
Gellam gum (GG) is a linear, negatively charged exopolysaccharide, also called as S-60 [49]. A bacterial polysaccharide called GG was initially made commercially by Kelco (now Monsanto PLC) using the bacterium Sphingomonas elodea. The de-esterification produces a stiff, brittle gel, while the native polysaccharide generates a weak, elastic gel [50]. At high temperatures, gellan molecules appear as random coils, but at low temperatures, they appear as double helices. GG can withstand acid and heat stress when being manufactured. It is ductile, non-toxic, biocompatible, biodegradable, and thermoresponsive. GG possesses mucoadhesive qualities. Due to its negative charge, this polysaccharide can create polyelectrolytes with polymers that have the opposite charge, such as chitosan. GG is regarded as a pseudoplastic at high shear rates. GG is not destroyed by an acidic environment and is resistant to enzymatic activity. The GG beads expand at high pH levels and remain stable at low pH levels. Additionally, it possesses a broad range of mechanical, acceptable rheological, and high processability qualities. The finest qualities of GG are its high efficiency, malleability, and gelling ability [49].
5. Biomedical Applications of Polysaccharide-Based Implantable Devices
5.1. Implants for Oral Cavity
5.2. Implants for Nasal Cavity
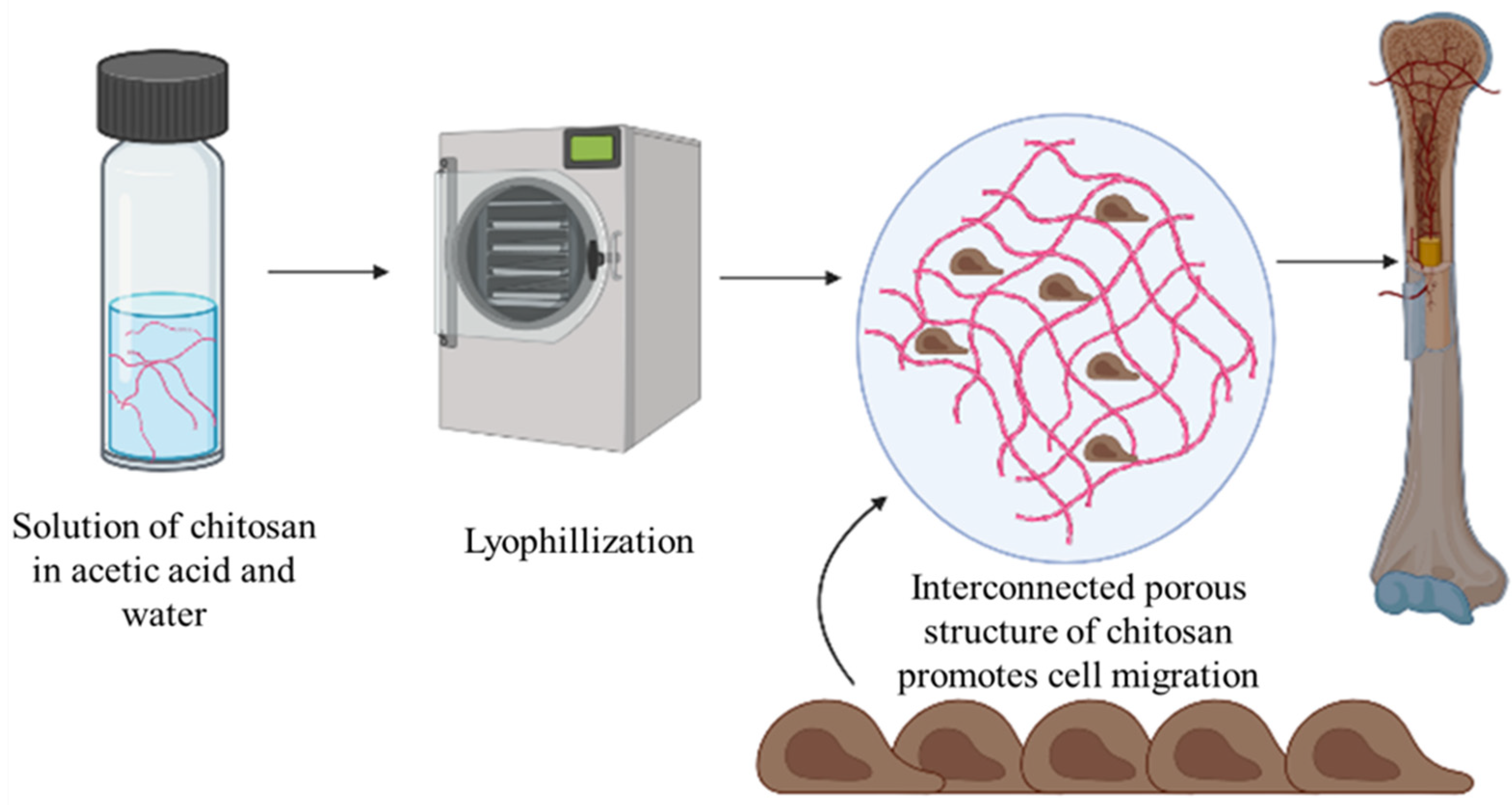
5.3. Bone Implants
5.4. Implant for Ocular Use
Proteins and polysaccharides appear as ideal candidates for biodegradable drug delivery due to their biocompatibility, biodegradability, low immunogenicity, and pH stability under physiological conditions. Polysaccharides such as cellulose, hyaluronic acid, gelatin, collagen, xanthan gum, alginic acid, and chitosan have been successfully explored in drug delivery for eye diseases. These polysaccharides are extensively used as additives for improvements in permeability, contact time, and ocular absorption. Chitosan is the most widely explored polymer for ocular drug delivery due to its mucoadhesive property and inertness [64]. Manna et al. prepared intravitreal chitosan and polylactic acid-based methotrexate micro-implants to treat primary intraocular lymphoma. The results indicate that uncoated chitosan methotrexate implants administer drug approximately for 1 day, and after coating with polylactic acid, the implants show drug release for 50 days with a release rate of 0.2–2.0 µg/day [65]. In further continuation of their previous work, to improve the methotrexate release profile, Manna et al. utilized different combinations of PLGA-PLA coating. They observed two findings after the increase in the PLA content in PLGA: (a) the initial burst release effect gets reduced, and (b) delayed swelling and biodegradation of the micro implants. After coating with different ratios of PLA-PLGA, they observed drug release of 0.2–2.0 µg/day of methotrexate for an extended period of ∼3–5 months [66].
5.5. Implants for Antiviral Therapy
The major limitation associated with antiretroviral therapy is its longer duration of treatment, leading to nonadherence to the medication [67]. In order to overcome these limitations, long-acting antiviral drug-loaded biodegradable implants have been developed which can offer sustained release of the antiretroviral drug for a considerable period of time, ranging from several weeks to months [68]. Though, the utilization of polysaccharide based coated implants for antiretroviral therapy is not yet established in the literature, various polysaccharides including heparin, galactan, fucoidan, glucan, cellulose, dextran, or dextrin have been reported to possess antiviral properties, that can be explored in the future for prevention of viral infections [69]. The layer-by-layer coating of polysaccharides on the implant surface can be used as a potential approach to prevent viral growth on implants [70]. The ability of sulfated polysaccharides to mimic the glycosaminoglycans present in the cell membrane confers it with distinct antiviral properties. Sulfated polysaccharides are known to interfere with the steps involved in the lifecycle of a virus such as adsorption, invasion, transcription, and replication and thus lead to an enhanced host immune response by accelerating the viral clearance rate. Hence, they offer a potential for further scientific and clinical research on implantable systems [71].
6. Conclusions
The use of polysaccharide-based implantable devices in the treatment of various diseases is becoming increasingly important. Polysaccharides are used in the development of implantable devices to improve its biodegradability and biocompatibility. In addition, polysaccharides also confer certain unique properties to the composites such as mechanical strength, which favors the tissue reconstruction process. Though only a few commercial products, as discussed in the previous sections, have been successfully developed, the scope of this field is emerging vastly and holds a promising potential to create a niche market. In recent times, a considerable number of efforts have been devoted towards the development of biodegradable polysaccharide implants by various researchers and it can be expected in the near future that these innovative composites can undergo scale-up and commercialization. This would serve as a breakthrough achievement in the field of biomedical sciences, thus expanding the scope of tissue engineering applications. Polysaccharide-based implanted devices or coated implants outperform synthetic or semi-synthetic polymers. Polysaccharide-based devices are now being studied/explored for their physicochemical features, which include surface morphology, in-vitro characterization, and in-vitro evaluation. However, once implanted as a medical intervention, the implants begin to integrate the unique interaction with human body elements such as cells, tissue, organs, or the endocrine system. As a result, it is critical to comprehend such a potential interaction and research the side effects of those implantable devices. Because polysaccharides are widely used in biomedical and pharmaceutical applications, further examination is required due to safety concerns. Polysaccharide-based materials are now regarded safe in terms of biocompatibility, biodegradability, and non-toxicity, although additional research should be conducted. Once, the safety of these devices is well-established, it will in turn enhance the patient acceptability. Further, the degradation rate and the mechanism of degradation has to be well-studied for each polysaccharide. The erosion rate can significantly affect the drug release. Quick degradation in the physiological environment and result in excessive release of the drug (burst release) and may also result in premature loss of strength of the polysaccharide. Thus, the degradation mechanism of each polysaccharide needs to be validated and must be well controlled so that a better idea can be obtained regarding its in-vivo behavior.
This entry is adapted from the peer-reviewed paper 10.3390/polysaccharides3030037
References
- Dash, A.; Cudworth, G. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12.
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379.
- Kumar, A.; Pillai, J. Implantable Drug Delivery Systems: An Overview. In Nanostructures for the Engineering of Cells, Tissues and Organs From Design to Applications; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–511.
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, Their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21-5.
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243.
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloids Interface Sci. Commun. 2021, 42, 100418.
- Yang, Y.; Wu, H.; Fu, Q.; Xie, X.; Song, Y.; Xu, M.; Li, J. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 110394.
- López-Valverde, N.; López-Valverde, A.; Cortés, M.P.; Rodríguez, C.; De Sousa, B.M.; Aragoneses, J.M. Bone Quantification Around Chitosan-Coated Titanium Dental Implants: A Preliminary Study by Micro-CT Analysis in Jaw of a Canine Model. Front. Bioeng. Biotechnol. 2022, 10, 858786.
- Nganga, S.; Travan, A.; Marsich, E.; Donati, I.; Söderling, E.; Moritz, N.; Paoletti, S.; Vallittu, P.K. In vitro antimicrobial properties of silver–polysaccharide coatings on porous fiber-reinforced composites for bone implants. J. Mater. Sci. Mater. Electron. 2013, 24, 2775–2785.
- AlNufaiy, B.M.; Lambarte, R.N.A.; Al-Hamdan, K.S. The Osteogenetic Potential of Chitosan Coated Implant: An In Vitro Study. J. Stem Cells Regen. Med. 2020, 16, 44–49.
- McDonald, M.; D’Aversa, G.; Perry, H.D.; Wittpenn, J.R.; Donnenfeld, E.D.; Nelinson, D.S. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans. Am. Ophthalmol. Soc. 2009, 107, 214–221.
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236.
- Liu, H.; Ramsden, L.; Corke, H. Physical properties and enzymatic digestibility of hydroxypropylated ae, wx, and normal maize starch. Carbohydr. Polym. 1999, 40, 175–182.
- Adetunji, A.O. Chemically Modified Starches as Excipients in Pharmaceutical Dosage Forms. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020.
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and Chemical Modifications in Starch Structure and Reactivity. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020.
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Druzian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234.
- Hsieh, Y.-C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513.
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A Mater. Sci. Process. 2005, 80, 93–97.
- Brown, R.M.; Willison, J.H.; Richardson, C.L. Cellulose biosynthesis in Acetobacter xylinum: Visualization of the site of synthesis and direct measurement of the in vivo process. Proc. Natl. Acad. Sci. USA 1976, 73, 4565–4569.
- Trovatti, E.; Oliveira, L.; Freire, C.S.; Silvestre, A.; Neto, C.; Pinto, J.J.C.; Gandini, A. Novel bacterial cellulose–acrylic resin nanocomposites. Compos. Sci. Technol. 2010, 70, 1148–1153.
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491.
- Zimnitsky, D.S.; Yurkshtovich, T.L.; Bychkovsky, P.M. Synthesis and Characterisation of Oxidized Cellulose. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4785–4791.
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27, 3955–3963.
- Jeschke, M.G.; Sandmann, G.; Schubert, T.; Klein, D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen. 2005, 13, 324–331.
- Bassetto, F.; Vindigni, V.; Scarpa, C.; Botti, C.; Botti, G. Use of Oxidized Regenerated Cellulose to Stop Bleeding After a Facelift Procedure. Aesthetic Plast. Surg. 2008, 32, 807–809.
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712.
- Smidsrød, O.; Haug, A.; Larsen, B. The Influence of pH on the Rate of Hydrolysis of Acidic Polysaccharides. Acta Chem. Scand. 1966, 20, 1026–1034.
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389.
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546.
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462.
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44.
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid Interface Sci. 2019, 269, 296–308.
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583.
- De Ruiter, G.A.; Rudolph, B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395.
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa -Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Health Mater. 2012, 2, 895–907.
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.; Ramakrishna, S.; Berto, F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers 2021, 13, 1744.
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2014, 121, 27–36.
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Application of Compatibilized Polymer Blends in Biomedical Fields. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537.
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999.
- Tirtaatmadja, V.; Dunstan, D.E.; Boger, D.V. Rheology of dextran solutions. J. Non-Newtonian Fluid Mech. 2001, 97, 295–301.
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657.
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Effect of pH on the Behavior of Hyaluronic Acid in Dilute and Semidilute Aqueous Solutions. Macromol. Symp. 2008, 274, 131–140.
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411.
- Pisárčik, M.; Bakoš, D.; Čeppan, M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 97, 197–202.
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701.
- Training Manual on Gracilaria Culture and Seaweed Processing in China. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF9214331 (accessed on 28 July 2022).
- BeMiller, J.N. An Introduction to Pectins: Structure and Properties. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washington, DC, USA, 1986; pp. 2–12.
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104.
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2017, 109, 1068–1087.
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24.
- Carolina Ramirez Hernandez, F. Use of Hyaluronic Acid in Osteointegration of Dental Implants. Sci. Arch. Dent. Sci. 2019, 2, 27–28.
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670.
- López-Valverde, N.; López-Valverde, A.; Ramírez, J. Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants. Biology 2021, 10, 102.
- Kim, D.H.; Lee, H.H.; Kim, S.H.; Hwang, S.H. Effectiveness of using a bioabsorbable implant (Latera) to treat nasal valve collapse in patients with nasal obstruction: Systemic review and meta-analysis. Int. Forum Allergy Rhinol. 2020, 10, 719–725.
- Romo, T.; Pearson, J.M. Nasal Implants. Facial Plast. Surg. Clin. N. Am. 2008, 16, 123–132.
- Sclafani, A.P.; Romo, T.; Iii, M. Biology and Chemistry of Facial Implants. Facial Plast. Surg. 2000, 16, 3–6.
- Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges. Pharmaceutics 2014, 6, 249–267.
- Park, S.; Park, J.; Heo, J.; Lee, S.-E.; Shin, J.-W.; Chang, M.; Hong, J. Polysaccharide-based superhydrophilic coatings with antibacterial and anti-inflammatory agent-delivering capabilities for ophthalmic applications. J. Ind. Eng. Chem. 2018, 68, 229–237.
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent advances in biopolymeric composite materials: Future sustainability of bone-implant. Renew. Sustain. Energy Rev. 2021, 150, 111505.
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347.
- Lee, E.-J.; Kim, H.-E. Accelerated bony defect healing by chitosan/silica hybrid membrane with localized bone morphogenetic protein-2 delivery. Mater. Sci. Eng. C 2016, 59, 339–345.
- Agarwal, T.; Kabiraj, P.; Narayana, G.H.; Kulanthaivel, S.; Kasiviswanathan, U.; Pal, K.; Giri, S.; Maiti, T.K.; Banerjee, I. Alginate Bead Based Hexagonal Close Packed 3D Implant for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 32132–32145.
- Bhattacharjee, A.; Bose, S. 3D Printed Hydroxyapatite–Zn2+ Functionalized Starch Composite Bone Grafts for Orthopedic and Dental Applications. Mater. Des. 2022, 221, 110903.
- Marõ´a, M.; Alonso, J.; Sá Nchez, A.; Alonso, M.J.; Mara, M.; Sá, A. The Potential of Chitosan in Ocular Drug Delivery. J. Pharm. Pharmacol. 2010, 55, 1451–1463.
- Manna, S.; Augsburger, J.J.; Correa, Z.M.; Landero, J.A.; Banerjee, R.K. Development of Chitosan and Polylactic Acid Based Methotrexate Intravitreal Micro-Implants to Treat Primary Intraocular Lymphoma: An In Vitro Study. J. Biomech. Eng. 2014, 136, 021018.
- Manna, S.; Donnell, A.M.; Kaval, N.; Al-Rjoub, M.F.; Augsburger, J.J.; Banerjee, R.K. Improved Design and Characterisation of PLGA/PLA-Coated Chitosan Based Micro-Implants for Controlled Release of Hydrophilic Drugs. Int. J. Pharm. 2018, 547, 122–132.
- Weld, E.D.; Flexner, C. Long-acting implants to treat and prevent HIV infection. Curr. Opin. HIV AIDS 2020, 15, 33–41.
- Maturavongsadit, P.; Shrivastava, R.; Sykesc, C.; Cottrell, M.L.; Montgomery, S.A.; Kashuba, A.D.M.; Benhabbour, S.R. Biodegradable Polymeric Solid Implants for Ultra-Long-Acting Delivery of Single or Multiple Antiretroviral Drugs. Int. J. Pharm. 2021, 605, 120844.
- Rodrigues, I.C.P.; Campo, K.N.; Arns, C.W.; Gabriel, L.P.; Webster, T.J.; Lopes, É.S.N. From Bulk to Nanoparticles: An Overview of Antiviral Materials, Its Mechanisms, and Applications. Part. Part. Syst. Charact. 2021, 38, 2100044.
- Otto, D.P.; de Villiers, M.M. Layer-by-Layer Nanocoating of Antiviral Polysaccharides on Surfaces to Prevent Coronavirus Infections. Molecules 2020, 25, 3415.
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526.
