Functional beverages can be a valuable component of the human diet with the ability to not only provide essential hydration but to deliver important bioactive compounds that can contribute to chronic disease treatment and prevention. One area of the functional beverage market that has seen an increase in demand in recent years are beverages that promote relaxation and sleep. Sleep is an essential biological process, with optimal sleep being defined as one of adequate duration, quality and timing. It is regulated by a number of neurotransmitters which are, in turn, regulated by dietary intake of essential bioactive compounds.
- sleep
- nutraceuticals
- theanine
- chamomile extract
- tryptophan
- cysteine
- functional beverages
1. Introduction
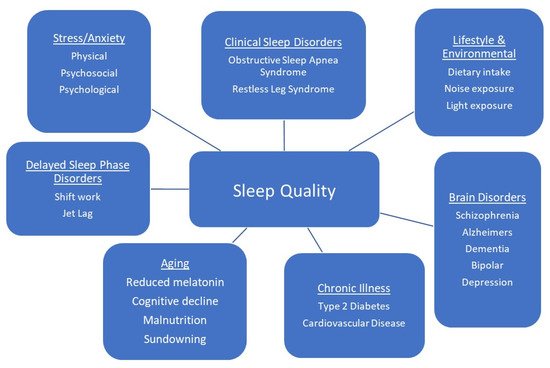
2. Active Compounds
|
Compound |
Reference/Country |
Participants |
Intervention/Duration |
Study Design |
Outcome Measures |
Effects on Sleep |
|---|---|---|---|---|---|---|
|
Markus et al. (2005) [40] Netherlands |
Adults without sleep complaints (n = 14) Age (22 ± 3 years) Adults with mild sleep complaint (n = 14) Age (22 ± 2 years) |
20 g L-TRP-enriched A-LAC protein (4.8 g L-TRP/100 g amino acids w/w) 1 night |
Double-blind Placebo-controlled |
Subjective Sleep Quality Measures: Stanford Sleepiness Scale |
Improved morning alertness (p = 0.013) and increased attention (p = 0.002) in both groups. Improved performance in participants with sleep complaints only (p = 0.05). |
|
|
L-Tryptophan |
Ong et al. (2017) [41] Australia |
Healthy males without sleep complaint (n = 10) Age (26.9 ± 5.3 years) |
20 g L-TRP-enriched A-LAC protein (4.8 g L-TRP/100 g amino acids w/w) of A-LAC protein 2 nights |
Double-blind Placebo-controlled Randomized Crossover |
Objective Sleep Quality Measures (Actigraphy): Total sleep time Sleep onset latency Sleep efficiency (%) Wake time after sleep onset Subjective Sleep Measures (Sleep Log): Bedtime Time taken to fall asleep Frequency of awakenings Time taken to return to sleep Waking time Rising time Total sleep time |
Increased objective and subjective total sleep time by 12.8% (p = 0.037) and 10.8% (p = 0.013), respectively; increased objective sleep efficiency by 7.0% (p = 0.028). |
|
Cubero et al. (2007) [42] Spain |
Pre-weaning infants (n = 30) Age (4–20 weeks) |
Diet A: Standard formula Diet B: Standard formula during the day and night formula (3.4 g L-TRP/100 g protein) Diet C: Day formula during the day (1.5 g L-TRP/100 g protein) + night formula (3.4 g L-TRP/100 g protein) in the evening 1 week per formula |
Double-blind Randomized |
Objective Sleep Quality Measures (Actigraphy): Time of nocturnal sleep Minutes of immobility Sleep latency Nocturnal awakenings Sleep efficiency (%) Sleep Diary: Sleep over 24 h Number of bottle feeds Observations or incidences that would influence the infants rest |
Diet C improved objective total sleep time (p < 0.05) and subjective (parent) sleep improvement; Diet B and Diet C reduced objective sleep onset latency; Diet B improved objective sleep efficiency. (All p’s < 0.05) |
|
|
Bravo et al. (2013) [43] Spain |
Older adults with sleep difficulties (n = 35) Age (55–75 years) |
L-TRP (60 mg) enriched cereal for breakfast and dinner 1 week |
Blind assay |
Objective Sleep Quality Measures (Actigraphy): Time in bed Assumed sleep Actual sleep time Sleep onset latency Sleep efficiency (%) Number of awakenings Immobile time Total activity Fragmentation index (indicator of quality of rest) |
Improvements in objective sleep measures including increase in actual sleep time (p < 0.01); increase in sleep efficiency (p < 0.001); increase in immobile time (p < 0.01); reduction in sleep latency (p < 0.01); wake bouts (p < 0.05); total activity (p < 0.01); fragmentation index (p < 0.001). |
|
|
5-HTP |
Bruni et al. (2004) [44] Italy |
Children with sleep terrors (n = 45) Age (3.2–10.6 years) |
2 mg/kg (Daily) 20 days |
Randomized, controlled |
Frequency of sleep terrors |
After 1-month: Sleep terrors reduced > 50% from baseline in 93.5% of children treated with 5-HTP (p < 0.00001). After 6 months: 51.6% were sleep-terror free (p < 0.001). |
|
Melatonin |
Scheer et al. (2012) [45] USA |
Hypertensive adults on beta blockers (n = 16) Age (45–64 years) |
2.5 mg (nightly, 1 h before bedtime) 3 weeks |
Randomized, Double-blind Placebo-controlled Parallel-group design |
Objective Sleep Quality Measures (Polysomnography): Sleep stages Total sleep time Time in bed Sleep efficiency (%) Objective Sleep Quality Measures (Actigraphy): Sleep onset latency Total sleep time Sleep efficiency (%) |
Increased total sleep time by 32 min (p = 0.046); increased sleep efficiency by 7.6% (p = 0.046). Decreased sleep onset latency to stage 2 NREM sleep by 14 min (p = 0.001) and increased the duration of stage 2 NREM sleep by 42 min (p = 0.037). |
|
Grima et al. (2018) [46] Australia |
Adults with sleep disturbance post onset of traumatic brain injury (n = 33) Age (37 ± 11 years) |
2 mg (nightly 2 h before bedtime) 4 weeks |
Randomized, Double-blind Placebo-controlled Two-period Two-treatment Crossover study |
Objective Sleep Quality Measures (Actigraphy) Sleep onset latency Total sleep time Sleep duration Sleep efficiency (%) Sleep Diary: Sleep onset/offset Sleep duration Subjective Sleep Quality Measures: PSQI ESS FSS |
Improved subjective sleep quality (p < 0.0001) and objective sleep efficiency (p < 0.04). |
|
|
Xu et al. (2020) [47] China |
Adults with primary insomnia (n = 97) Age (45–60 years) |
3 mg (nightly 1 h before bedtime) 4 weeks |
Randomized, Double-blind Placebo-controlled Parallel study |
Objective Sleep Quality Measures (Polysomnography): Sleep stages Total sleep time Sleep onset latency Wake after sleep onset Sleep efficiency (%) Subjective Sleep Quality Measures: PSQI ESS ISI |
Decreased objective sleep measures including early morning wake (p = 0.001) and decreased percentage of Stage 2 NREM sleep (p = 0.031). |
|
|
L-Cysteine |
Sadasivam et al. (2011) [48] India |
Adults with obstructive sleep apnea (n = 20) Age (53.1 ± 2.3 years) |
600 mg (Mucinac, Cipla), three times per day 30 days |
Randomized, Placebo-controlled |
Objective Sleep Quality Measures (Polysomnography): Sleep stages Total sleep time Sleep onset latency Wake after sleep onset Sleep efficiency (%) Sleep apnea Snoring Subjective Sleep Quality Measures: ESS |
Improvements in objective slow wave sleep as sleep percent time (p < 0.001) and sleep efficiency. (p < 0.05). Reduction in subjective Epworth Sleepiness Score (p < 0.001). |
|
Rao et al. (2019) [49] Japan |
Healthy adult males (n = 22) Age (27.5 ± 0.9 years) |
4 × 50 mg (nightly, 1 h before bedtime) 6 days |
Randomized, Double-blind Placebo-controlled Crossover trial |
Objective Sleep Quality Measures (Actigraphy): Time in bed Wake after sleep onset Sleep onset latency Sleep length Sleep efficiency (%) Subjective Sleep Quality Measures: Obstructive Sleep Apnea Inventory questionnaire |
Improvements in objective sleep measures including an increase in objective sleep efficiency (p < 0.047) and reduction in intermittent wakening (p < 0.044). Improvements in subjective sleep measures including feeling of recovery from exhaustion or fatigue scores (p < 0.042) and improvement in refreshed upon awakening scores (p < 0.014). |
|
|
L-Theanine |
Lyon et al. (2011) [50] Canada |
Boys with ADHD (n = 98) Age (8–12 years) |
2 × 100 mg (twice per day, morning and evening) 6 weeks |
Randomized, Double-blind Placebo-controlled Parallel trial |
Objective Sleep Quality Measures (Actigraphy): Wake after sleep onset Sleep onset latency Sleep length Nocturnal activity Sleep efficiency (%) Subjective Sleep Quality Measures: Pediatric Sleep Questionnaire |
Improved objective measures including sleep efficiency (p < 0.05), and reduced nocturnal activity (p < 0.05). |
|
Sarris et al. (2019) [51] Australia |
Adults with GAD (n = 46) Age (40.7 ± 15 years in TG; 32.2 ± 9.29 years in PG) |
225 mg (twice daily); increased to 450 mg (twice daily) if anxiety score did not reduce by ≥35% after 4 weeks 8 weeks |
Randomized, Double-blind Placebo-controlled Multi-center pilot study |
Subjective Sleep Quality Measures: ISI |
Improved subjective sleep satisfaction (p < 0.015); improvements in ISI scores for “difficulty in falling asleep” (p < 0.049); “Problems waking up too early” (p < 0.017); and “interference with daily functioning” (p = 0.030) in control. |
|
|
Hidese et al. (2019) [52] Japan |
Healthy Adults (n = 30) Age (48.3 ± 11.9 years) |
200 mg tablet daily before sleep 4 weeks |
Randomized, Double-blind Placebo-controlled Crossover trial |
Subjective Sleep Quality Measures: PSQI |
Improved subjective sleep quality (p < 0.013), reduced sleep onset latency, sleep disturbance and use of sleep medication (All p’s < 0.05). |
|
|
Vitamin B12 |
Mayer et al. (1996) [53] |
Healthy Adults (n = 20) Age (CB12 = 36.6 ± 5.2 years. MB12 = 36.2 ± 5.2 years) |
3 mg (cyano-(CB12) or methylcobalamin (MB12)) 14 days |
Randomized Single-blind Between subject’s design |
Objective Sleep Quality Measures (Actigraphy): Wake after sleep onset Sleep onset latency Sleep length Nocturnal activity Sleep efficiency (%) Subjective Sleep Quality Measures: Morning and Evening VAS |
Reduction in objective sleep time (p = 0.036) in MB12 group improvements in sleep quality and daytime alertness (All p’s < 0.05). |
|
Luboshitzky et al. (2002) [54] Israel |
Healthy Adult Males (n = 12) Age (22–26 years) |
100 mg (5.00 PM) Once |
Randomized Placebo-controlled Parallel trial |
Objective Sleep Quality Measures (EEG): Sleep stages (%) Total recording time Sleep latency Actual sleep time Sleep efficiency (%) REM latency |
No effect. |
|
|
Vitamin B6 |
Ebben et al. (2002) [55] USA |
Healthy Adults (n = 12) Age (18–28 years) |
100 mg 250 mg Placebo (All nightly before bed) 5 days per treatment |
Placebo-controlled Double-blind Crossover trial |
Subjective Sleep Quality Measures: Sleep questionnaire Dream Salience Scale |
Increase in dream salient scores in 250 mg B6 treatment compared to placebo (p = 0.05). |
|
Aspy et al. (2018) [56] Australia |
Healthy Adults (n = 100) Age (mean = 27.5) |
120 mg (pyridoxine hydrochloride) Vitamin B Complex (120 mg pyridoxine hydrochloride + other B vitamins) Placebo (All nightly before bed) 5 days |
Randomized Double-blind Placebo-controlled trial |
Subjective Sleep Quality Measures: Sleep log |
Increased the amount of dream content recalled (p = 0.032) and decrease in sleep quality (p = 0.014) in B complex group. |
|
|
Vitamin D |
Ghaderi et al. (2017) [57] Iran |
Adults undergoing Methadone Treatment. (n = 68) Age (25–70 years) |
50,000 IU (once per fortnight) 12 weeks |
Randomized Double-blind Placebo-controlled trial |
Subjective Sleep Quality Measures: PSQI |
Improvement in subjective sleep score (p = 0.02). |
|
Mason et al. (2016) [58] USA |
Overweight menopausal females with low VitD (n = 218) Age (50–75 years) |
2000 IU vitamin D3 (daily) 12 months |
Randomized Double-blind Placebo-controlled trial |
Subjective Sleep Quality Measures: PSQI |
Increase in PSQI score (p = 0.01) and increase in need to take sleep medication (p < 0.01). |
|
|
Vitamin C |
Dadashpour et al. (2018) [59] Iran |
Adults on hemodialysis with sleep disorder (n = 90) Age (18–70 years) |
500 mg /5 cc intravenously–3 times per week 8 weeks |
Randomized Double-blind Trial |
Subjective Sleep Quality Measures: PSQI VAS |
Reductions in subjective sleep quality, sleep latency, daytime dysfunction (All p’s = 0.001). |
|
Yeom et al. (2007) [60] Korea |
Adults with Stage IV cancer (n = 39) Age (53.5 ± 10.5 years) |
10 g vitamin C intravenously twice with 3-day interval, then 4 g oral supplement daily 1 week |
Prospective study |
Subjective Sleep Quality Measures: European Organization for Research and Treatment of Cancer Core Quality-of-Life questionnaire (EORTC QLQ-C30)-Korean Version |
Lower subjective scores for sleep disturbance and fatigue (p < 0.005). |
|
|
Murck et al. (2000) [61] Germany |
Older adults without sleep disturbances (n = 12) Age (60–80 years) |
10 mmol for 3 days, then 20 mmol for 3 days, then 30 mmol daily for 14 days |
Randomized Placebo-controlled Crossover design |
Objective Sleep Quality Measures (EEG): Sleep stages (%) Total recording time Sleep latency Actual sleep time Sleep efficiency (%) REM latency |
Increase in slow wave sleep (p < 0.05), delta and sigma waves (p < 0.05 for both). |
|
|
Magnesium |
Abbasi et al. (2012) [62] Iran |
Older adults (n = 43) Age (65 ± 4.6 years) |
414 mg magnesium oxide (250 mg Mg) Twice per day 8 weeks |
Double-blind Placebo-controlled trial |
Subjective Sleep Quality Measures: ISI Sleep Log |
Increase in subjective sleep time (p = 0.002) and subjective sleep efficiency (p = 0.03); decrease in subjective sleep onset latency (p = 0.04), and insomnia severity index (p = 0.006). |
|
Hornyak et al. (2004) [63] Germany |
Alcohol dependent adults in subacute withdrawal with sleep disturbance (n = 11) |
30 mmol Magnesium L-aspartate hydrochloride (10 mmol morning and 20 mmol evening) daily 4 weeks |
Open Pilot Study |
Objective Sleep Quality Measures (Polysomnography): Sleep stages Total sleep time Sleep onset latency Wake after sleep onset Sleep efficiency (%) Periodic leg movements in sleep (PLMS) Subjective Sleep Quality Measures: PSQI |
Decrease in objective sleep latency (p = 0.03), improvement in subjective sleep quality (p = 0.05). |
|
|
Zinc |
Saito et al. (2017) [64] Japan |
Healthy Adults (n = 94) Age (20–84 years) |
Group A: Placebo Group B: 15 mg Group C: 15 mg + Astx Group D: Placebo + 16 mg + Astx 12 weeks |
Randomized Double-blind Placebo-controlled Parallel group trial |
Objective Sleep Quality Measures (Actigraphy): Wake after sleep onset Sleep onset latency Sleep length Frequency Nocturnal activity Sleep efficiency (%) Subjective Sleep Quality Measures: PSQI |
Improvements in objective sleep efficiency in group B (p = 0.025); objective sleep onset latency in Group B and D (p < 0.032) and (p = 0.004), respectively. |
|
Gholipour et al. (2018) [65] Iran |
ICU nurses (n = 54) Age (31.2 ± 5.42 years) |
1 × 220 mg (every 72 h) 1 month |
Multi-center Randomized Two parallel group Placebo-controlled trial |
Subjective Sleep Quality Measures: PSQI |
Improvements in subjective total sleep quality (p < 0.002); sleep onset latency (p < 0.003), sleep duration (p < 0.02) and total sleep quality score (p < 0.008). |
Note: L-TRP-L-Tryptophan; A-LAC–alpha-lactalbumin; EEG–Electroencephalography; 5-HTP–5-hydroxytryptophan; SWS–Slow Wave Sleep; REM–Rapid Eye Movement; NREM–Non-Rapid Eye Movement; ADHD–Attention Deficit Hyperactivity Disorder; GAD–Generalized Anxiety Dissorder; TG–Treatment Group; PG–Placebo Group; PSQI–Pittsburgh Sleep Quality Index; ICU–Intensive Care Unit; ESS–Epworth Sleepiness Scale; FSS–Fatigue Severity Scale; ISI–Insomnia Severity Index; VAS–Visual Analogue Scale; Astx-Astaxanthin.
3. Discussion
This entry is adapted from the peer-reviewed paper 10.3390/beverages7020033
