Efficacy evaluation through a disease model using the gut microbiome of well-controlled obese rats using a risk prediction model based on clinical microbiome data for inflammatory bowel disease and colorectal cancer is a useful food efficacy evaluation tool.
- Microbiology
- Metagenomics
- Dietary efficacy
- Risk assessment
- Fecal microbiome
- Nypa fruticans
- Gut disease
- 16S amplicon sequencing
- obesity
1. Introduction
Although obesity has been studied to influence chronic inflammatory diseases, the causal relationship between inflammatory diseases and the microbiome has not been clearly established. Based on the clinical microbiome information in colorectal disease, the association of the obesity model was confirmed, and furthermore, the potential as a food efficacy evaluation tool was confirmed based on the risk prediction model.
1.1. Obesity and inflammation
For a long time, obesity has become a problem for everyone as the western diet, which includes a diet high in sugar and high fat, has become common. The proportion of overweight adults in many countries has increased in both developed and developing countries, and is a global epidemic with no country reporting a decrease in its incidence[1]. It has been found that obesity accelerates metabolic syndrome and leaves people more susceptible to chronic local inflammation[2]. This means that obesity changes the micro-environment at multiple levels throughout the body.
1.2. Microbiome research method
Recently, as research on microbial communities has been developed, amplicon sequencing using 16S ribosomal RNA has been widely used in addition to microbial culture. Due to the advancement of technology, the cost of sequencing data has been reduced, and it has become possible to obtain microbiome information in various environments. However, since such microbiome information does not actually link a causal relationship, it does not provide a pathological cause, and there is a limitation that the interaction between microorganisms is difficult to interpret. Therefore, there is a need for a study to derive the significance between the disease and the microbiome through a new approach.
1.3. Microbiome and colorectal disease
Inflammatory bowel disease is a well-known risk factor for the development of colorectal cancer, and it has been observed that there is a defect in the epithelial barrier and increased intestinal permeability[3]. Clinical studies have reported that the gut microbiome of patients with inflammatory bowel disease and colorectal cancer differs from that of healthy controls, suggesting that the gut microbiome may be a contributing factor in the initiation and development of this cancer[4],[5].
1.4. Risk assessment model
Human microbiome studies have often been conducted to predict and understand composition and function through host sequencing and microbiome sequencing. Past studies have used t-tests, ANOVAs, and Mann-Whitney U tests to assess increases or decreases in abundance in microbiome, and logistic regression analysis was used to classify host phenotypes. Although these statistical methods are still useful, the use of historical statistical methods without determining the distribution of the microbiome data or the characteristics of the disease has limitations in modern data analysis. Additionally, public databases that classify microorganisms through the V3-V4 regions of 16S rRNA do not clearly distinguish between species and subspecies levels, and assigns loosely assignments at the genus level. Therefore, a recent research trend is interested in performing precise predictive analysis on microbiome data by utilizing machine learning algorithms. The merging of machine learning and controlled disease models is a new evaluation indicator in the interpretation of clinical information.
2. Microbiome Comparison and Classification Model
The fecal microbiome pattern of inflammatory bowel disease and colorectal cancer was compared with the fecal microbiome of the normal group. A classification model was developed to classify normal people and disease groups through two disease models (Figure 1, 2 and 3).
2.1. Microbiome of healthy people and patients with IBD
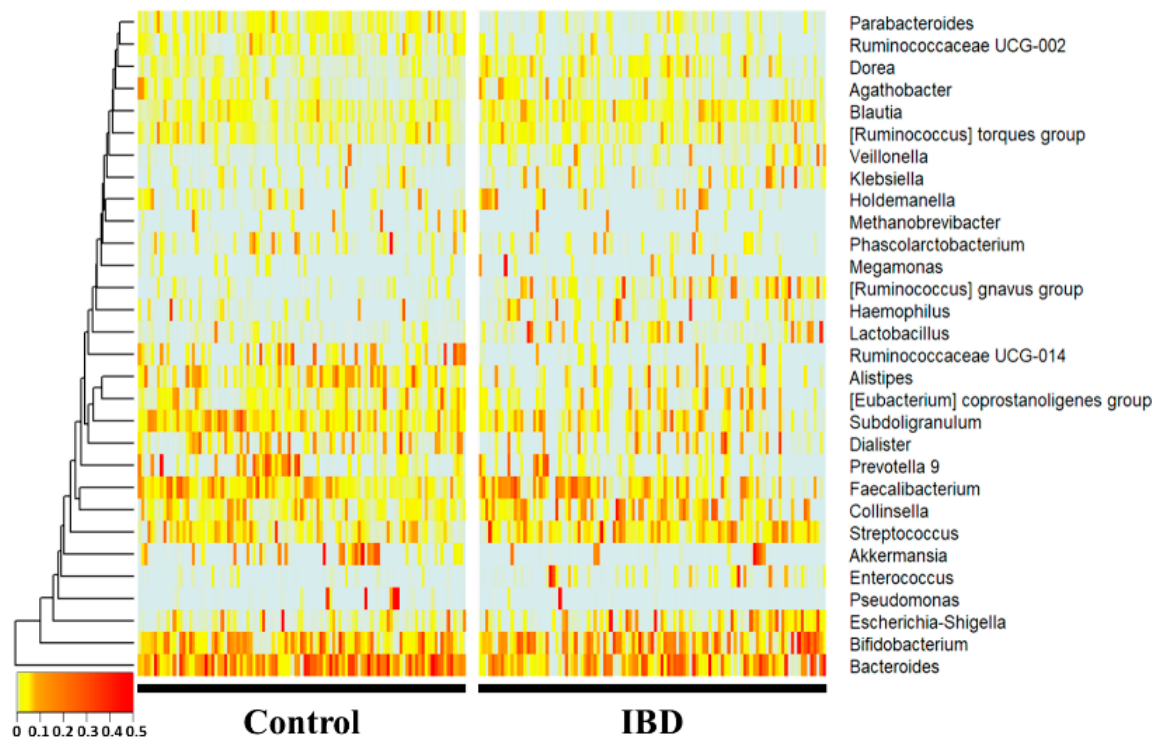
Figure 1. 16S amplicon sequencing was performed using feces of normal persons and inflammatory bowel disease patients. The comparison was performed at the genus level, which is easy to classify in the 16S metagenome analysis, and each person was placed on the horizontal axis and compared with the normal person and the inflammatory bowel disease group using a heat map. Statistically, Bacteroides, Subdoligranulum, Alistipes, Ruminococcaceae UCG-014, Parab-acteroides, and Ruminococcaceae UCG-002 were significantly reduced in the IBD group. Collinsella, Blautia, [Ruminococcus] gnavus group, Lactobacillus, and Dorea were significantly increased in the IBD group.
2.2. Microbiome of healthy people and CRC patients
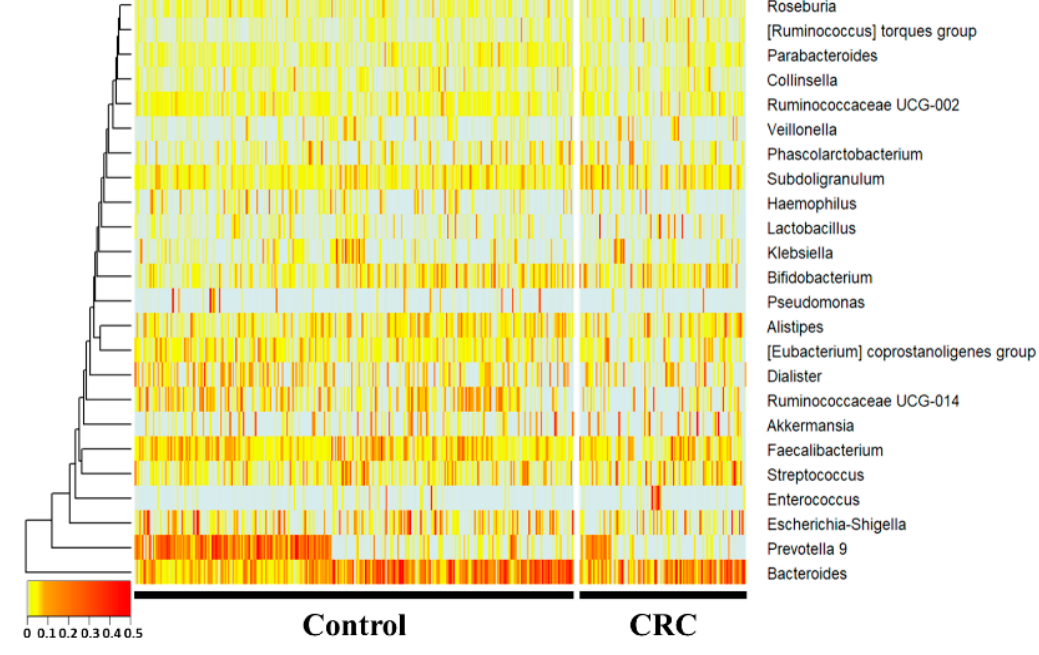
Figure 2. 16S amplicon sequencing was performed using feces of normal individuals and colorectal cancer patients. In the 16S metagenome analysis, comparisons were made at the genera level, which is easy to classify, and one person placed on the horizontal axis was compared to the normal person and colorectal cancer group using a heatmap. Statistically, Prevotella 9, Ruminococcaceae UCG-014, Haemophilus, and Parabacteroides were significantly reduced in the CRC group. Subdoligranulum and the [Ruminococcus] torques group were significantly increased in the CRC group.
2.3. Disease risk assessment model development
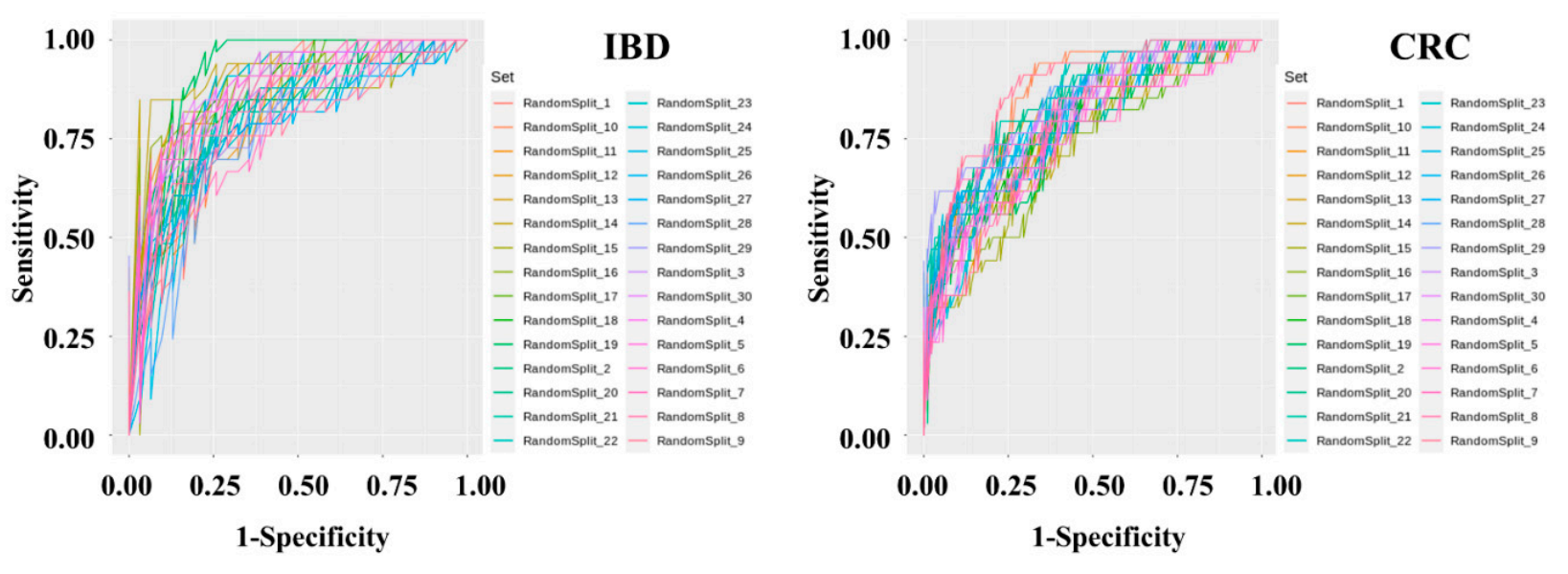
Figure 3. Metagenomic data preprocessing and machine learning were performed using feces of 395 normal persons, 109 IBD patients, and 111 CRC patients. After data preprocessing, a classification model was produced using the Gradient Boosting Machine technique. To solve the problem of overfitting the classification model, cross-validation was applied in which the data were randomly split into training and test sets in a 7:3 ratio for 30 iterations.
3. Establishment of Dietary Assessment Using Controlled Animal Models
An animal model to which the classification model obtained based on the clinical microbiome can be applied was selected. Since obesity is fundamentally associated with chronic inflammatory diseases, a high-fat diet model was selected. The perennial plant, Nypa fruticans (NF) Wurmb, is native to coastal mangrove areas. Vinegar made by fermenting the sap of this plant is used as a folk remedy for diabetes in Malaysian society. Two types of Nypa fruticans ethanol extract (NFE) and Nypa fruticans water extract (NFW) were selected for the development of dietary evaluation (Figure 4, 5 and 6).
3.1. Animal model evaluation
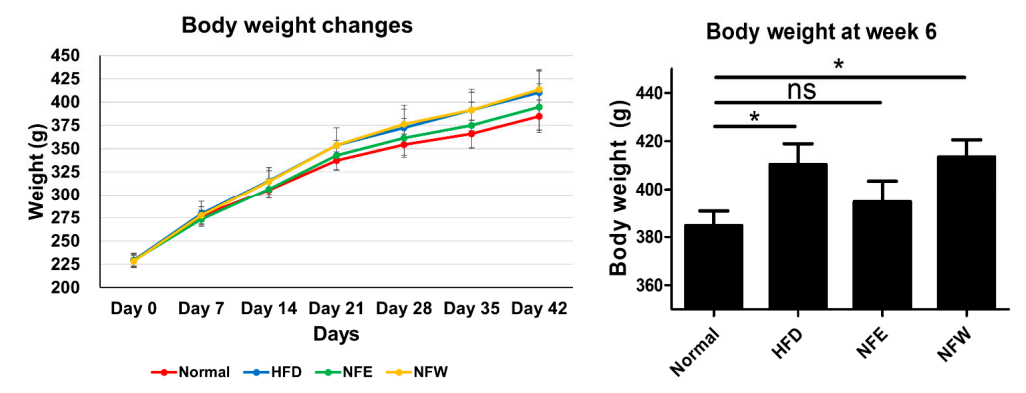
Figure 3. Rats were fed a high-fat diet for 6 weeks, and a normal control group was included. Weight change was confirmed by ingesting NFE and NFW at the same time as high-fat diet. The group that ate the high-fat diet was found to have statistical significance compared to the control group that did not, and there was no statistical difference in body weight between the normal group and the NFE.
3.2. Microbiome comparison between groups
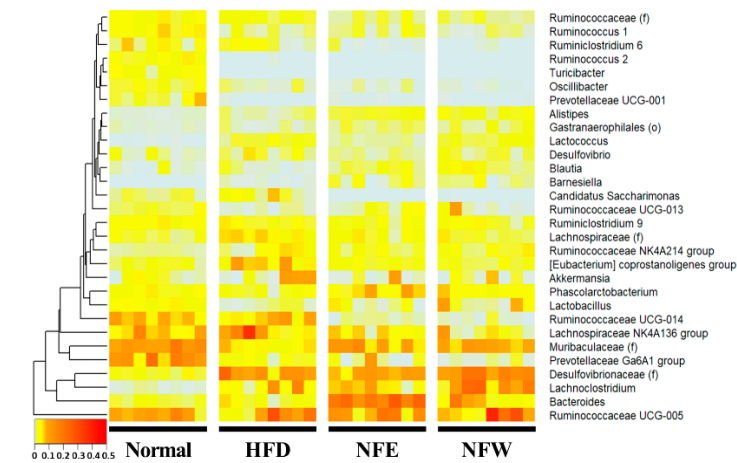
Figure 3. Metagenomic analysis was performed by collecting feces from rats. Comparison at the genera level, which is easy to classify in the 16S metagenome analysis, was performed, and one rat placed on the horizontal axis was listed and the heatmap was used for normal, high-fat diet, NFE with high-fat diet, and NFW with high-fat diet. and compared. Statistically, [Desulfovibrionaceae], Lachnoclostridium, [Eubacterium] coprostanoligenes group, and [Lachnospiraceae] were significantly reduced in the high-fat diet group. [Muribaculaceae], Prevotellaceae Ga6A1 group, Lactobacillus, [Ruminococca-ceae], Ruminococcus 1, and Ruminococcaceae UCG-013 were significantly increased in the high-fat diet group.
3.3. External validation and dietary efficacy evaluation
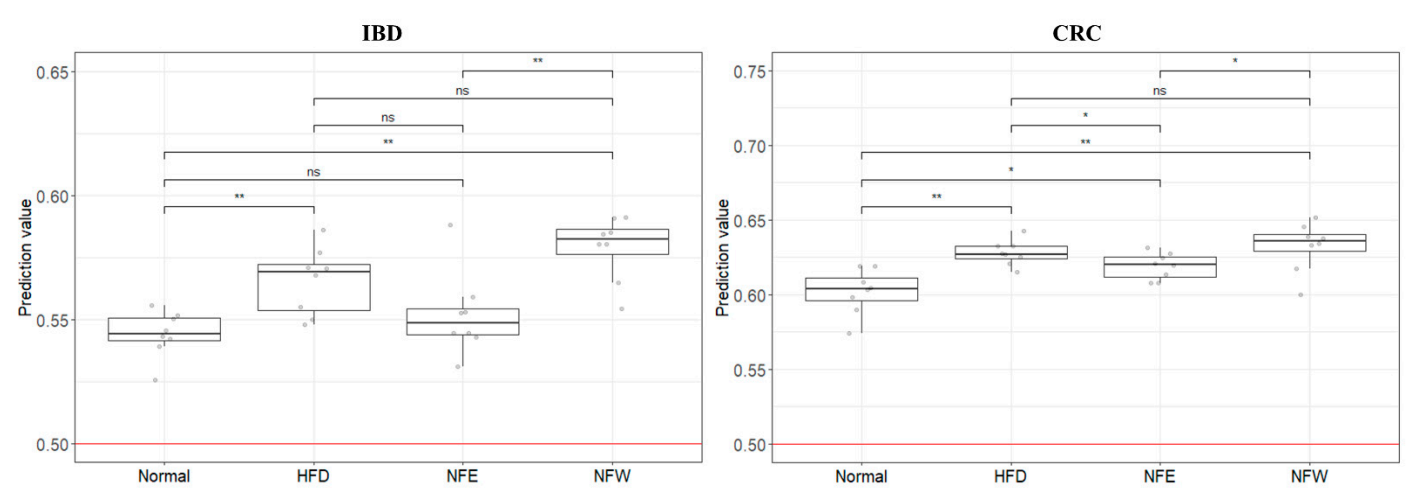
Figure 3. In order to evaluate the performance of the two models (inflammatory bowel disease model, colorectal cancer model) developed using clinical metagenomic data, the microbiome data of rats prepared above was used and applied to the GBM classification model. As a result, it was confirmed that the risk (0 = normal group, 1 = disease group) of the rat group fed a high-fat diet increased in both models compared to the normal group. It can be confirmed that the score of the disease group (inflammatory bowel disease, colorectal cancer) was higher than that of the normal group, and that the score decreased or increased through the intake of two types of food (NFE, NFW).
4. Food Efficacy Evaluation using Clinical Microbiome Data
Although typical studies compare microbiome data between groups through metagenome analysis, external verification is not easy to control[6]. To break this hurdle, clinical microbiome data are applied to well-controlled animal models. Although the application to the rat animal model based on the human-derived fecal microbiome profile is a limitation of the study, it is clear that it can be an accessible and more accessible evaluation model.
In addition, although it is an unclear attempt to apply the microbiome profile of inflammatory disease and cancer patients to a high-fat diet disease model, it is important to try based on the inflammation-microbiome correlation. Nevertheless, the fact that the risk was higher in the disease group than in the normal group also disproves the influence of the microbiome.
It can be demonstrate that risk prediction models generated based on the microbial profile of clinical disease can be externally validated utilizing appropriate animal disease models. Furthermore, it suggests that well-assessed microbiome-based risk assessment models are convenient tools for rapidly estimating the efficacy of diets and foods.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091833
References
- Marie Ng; Tom Fleming; Margaret Robinson; Blake Thomson; Nicholas Graetz; Christopher Margono; Erin C Mullany; Stan Biryukov; Cristiana Abbafati; Semaw Ferede Abera; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2014, 384, 766-781, 10.1016/S0140-6736(14)60460-8’.
- Eugenia E. Calle; Rudolf Kaaks; Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer 2004, 4, 579-591, 10.1038/nrc1408.
- Yi-Zhen Zhang; Inflammatory bowel disease: Pathogenesis. World Journal of Gastroenterology 2014, 20, 91-9, 10.3748/wjg.v20.i1.91.
- Kerri L. Glassner; Bincy P. Abraham; Eamonn M.M. Quigley; The microbiome and inflammatory bowel disease. Journal of Allergy and Clinical Immunology 2020, 145, 16-27, 10.1016/j.jaci.2019.11.003.
- Ester Saus; Susana Iraola-Guzmán; Jesse R. Willis; Anna Brunet-Vega; Toni Gabaldón; Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Molecular Aspects of Medicine 2019, 69, 93-106, 10.1016/j.mam.2019.05.001.
- Varsha Dave Badal; Dustin Wright; Yannis Katsis; Ho-Cheol Kim; Austin D. Swafford; Rob Knight; Chun-Nan Hsu; Challenges in the construction of knowledge bases for human microbiome-disease associations. Microbiome 2019, 7, 1-15, 10.1186/s40168-019-0742-2.