Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light. Structurally, pentacene is one of the linear acenes, the previous one being tetracene (four fused benzene rings) and the next one being hexacene (six fused benzene rings). In August 2009, a group of researchers from IBM published experimental results of imaging a single molecule of pentacene using an atomic force microscope. In July 2011, they used a modification of scanning tunneling microscopy to experimentally determine the shapes of the highest occupied and lowest unoccupied molecular orbitals. In 2012, pentacene-doped p-terphenyl was shown to be effective as the amplifier medium for a room-temperature maser.
- polycyclic
- linearly-fused
- p-terphenyl
1. Synthesis

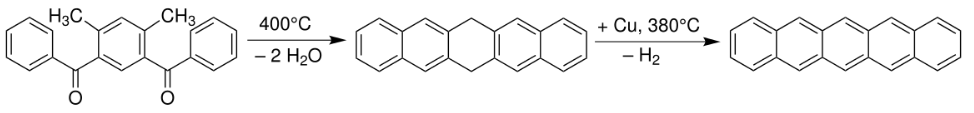
Pentacene was first synthesized in 1912 by British chemists William Hobson Mills and Mildred May Gostling.[2][3] A classic method for pentacene synthesis is by the Elbs reaction.[4][5]
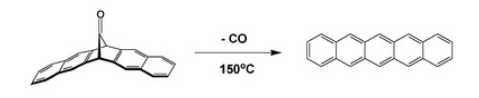
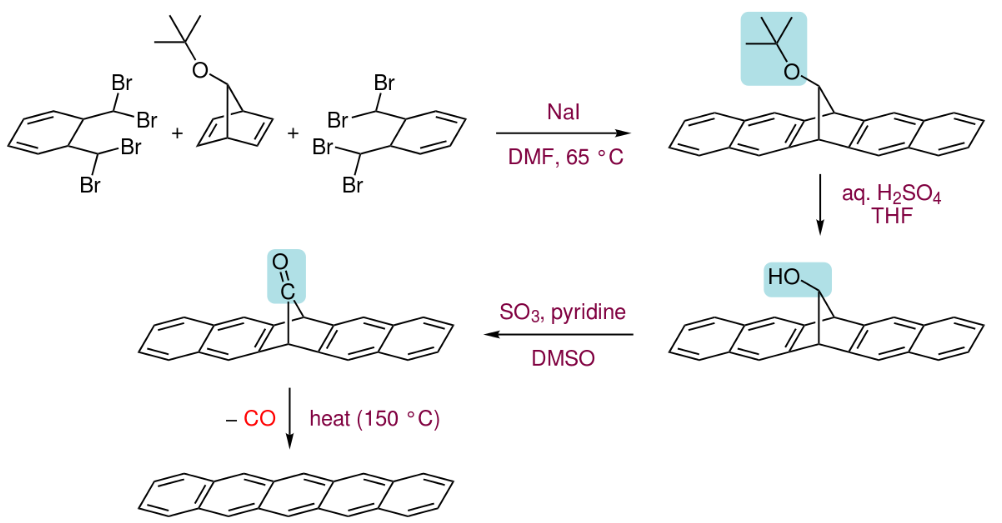
Pentacenes can also be prepared by extrusion of a small volatile component (carbon monoxide) from a suitable precursor at 150 °C.[6]
The precursor itself is prepared in three steps from two molecules of α,α,α',α'-tetrabromo-o-xylene with a 7-tert-butoxybicyclo[2.2.1]hepta-2,5-diene by first heating with sodium iodide in dimethylformamide to undergo a series of elimination and Diels–Alder reactions to form the ring system, then hydrolysing the tert-butoxy group to an alcohol and followed by its oxidation to the ketone.[6]
The product is reported to have some solubility in chloroform and is therefore amenable to spin coating. Pentacene is soluble in hot chlorinated benzenes, such as 1,2,4-trichlorobenzene, from which it can be recrystallized to form platelets.
2. Pentacene Derivatives
2.1. Monomeric Pentacene Derivatives
6,13-Substituted pentacenes are accessible through pentacenequinone by reaction with an aryl or alkynyl nucleophile (for example Grignard or organolithium reagents) followed by reductive aromatization.[7][8][9] Another method is based on homologization of diynes by transition metals (through zirconacyclopentadienes) [10][11][12][13][14] Functionalization of pentacene has allowed for control of the solid-state packing of this chromophore.[15][16] The choice of the substituents (both size and location of substitution on the pentacene) influences the solid-state packing and can be used to control whether the compound adopts 1-dimensional or 2-dimensional cofacial pi-stacking in the solid-state, as opposed to the herringbone packing observed for pentacene.
Although pentacene's structure resembles that of other aromatic compounds like anthracene, its aromatic properties are poorly defined; as such, pentacene and its derivatives are the subject of much research.
A tautomeric chemical equilibrium exists between 6-methylene-6,13-dihydropentacene and 6-methylpentacene.
-
6-methylpentacene equilibrium.
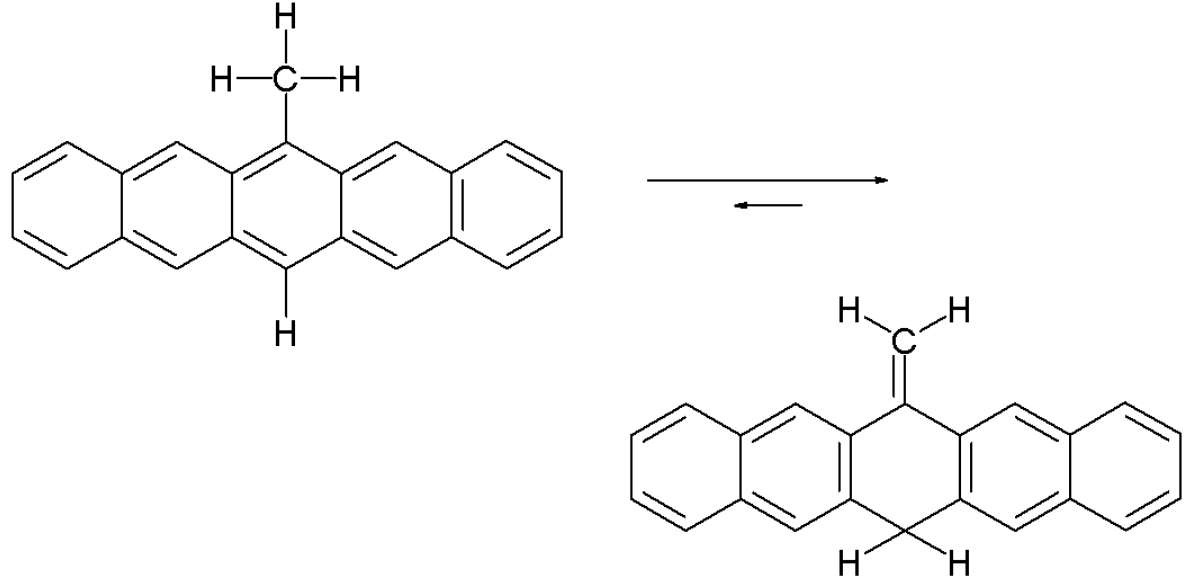
This equilibrium is entirely in favor of the methylene compound. Only by heating a solution of the compound to 200 °C does a small amount of the pentacene develop, as evidenced by the emergence of a red-violet color. According to one study[17] the reaction mechanism for this equilibrium is not based on an intramolecular 1,5-hydride shift, but on a bimolecular free radical hydrogen migration. In contrast, isotoluenes with the same central chemical motif easily aromatize.
Pentacene reacts with elemental sulfur in 1,2,4-trichlorobenzene to the compound hexathiapentacene.[18] X-ray crystallography shows that all the carbon-to-sulfur bond lengths are roughly equal (170 pm); from this, it follows that resonance structures B and C with complete charge separation are more significant than structure A.
-
Hexathiapentacene. By V8rik at the English-language Wikipedia, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=16246555
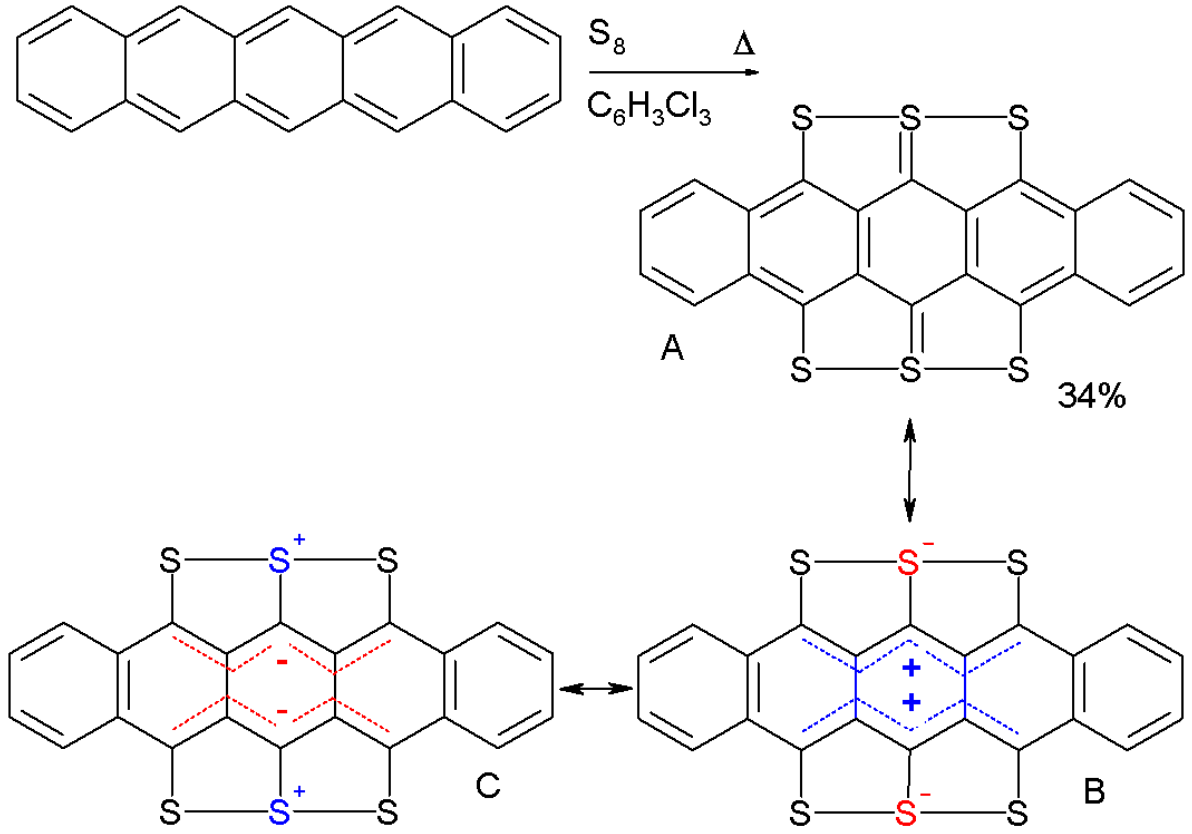
In the crystal phase the molecules display aromatic stacking interactions, whereby the distance between some sulfur atoms on neighboring molecules can become less (337 pm) than the sum of two Van der Waals radii (180 pm)
Like the related tetrathiafulvalene, this compound is studied in the field of organic semiconductors.
The acenes may appear as planar and rigid molecules, but in fact they can be very distorted. The pentacene depicted below:[19]
-
Twisted acenes.
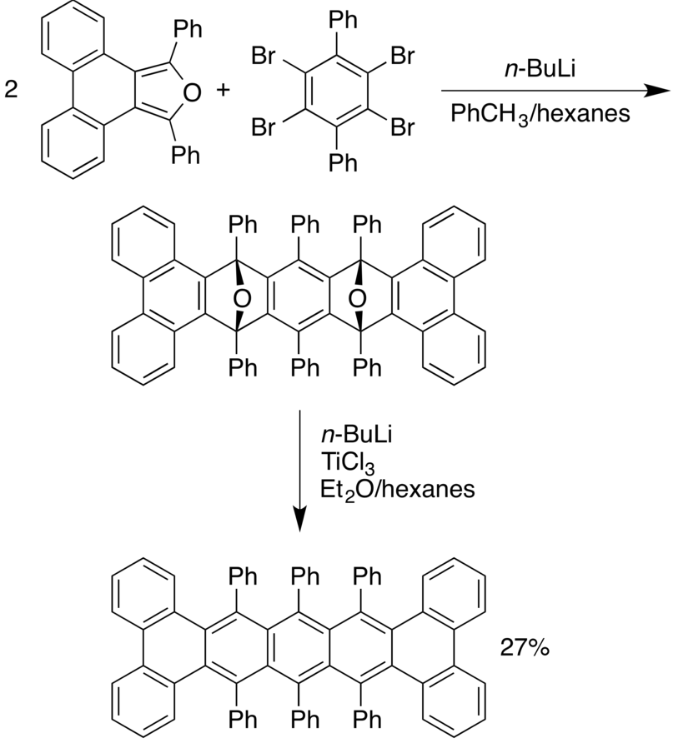
has an end-to end twist of 144° and is sterically stabilized by the six phenyl groups. The compound can be resolved into its two enantiomers with an unusually high reported optical rotation of 7400° although racemization takes place with a chemical half-life of 9 hours.
2.2. Oligomers and Polymers of Pentacene
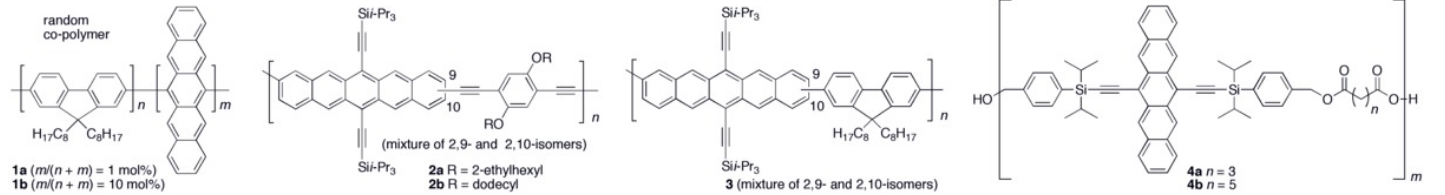
Oligomers and polymers based on pentacene have been explored both synthetically as well as in device application settings.[20][21] Polymer light emitting diodes (PLEDs) have been constructed using conjugated copolymers (1a–b) containing fluorene and pentacene.[22] A few other conjugated pentacene polymers (2a–b and 3) have been realized based on Sonogashira and Suzuki coupling reactions of a dibromopentacene monomer.[23][24] Non-conjugated pentacene-based polymers have been synthesized via esterification of a pentacene diol monomer with bis-acid chlorides to form polymers 4a–b.[25][26]
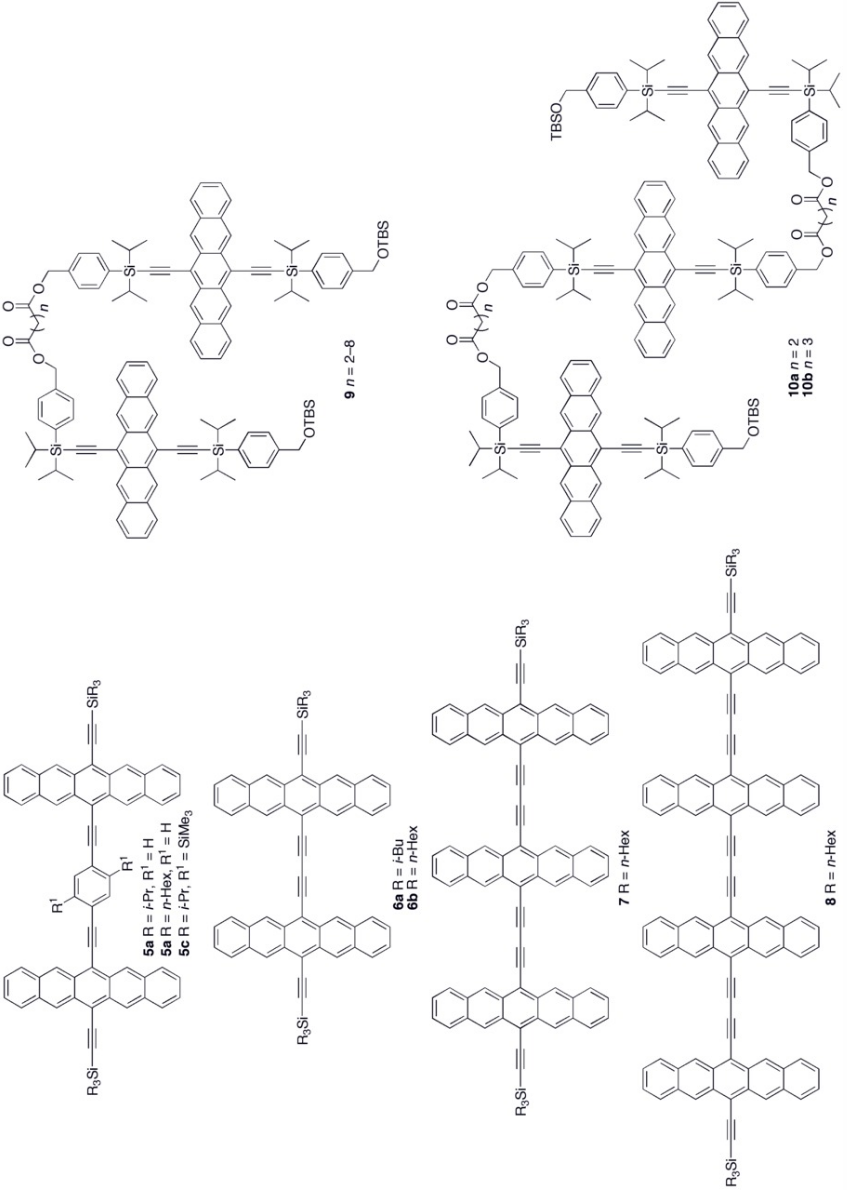
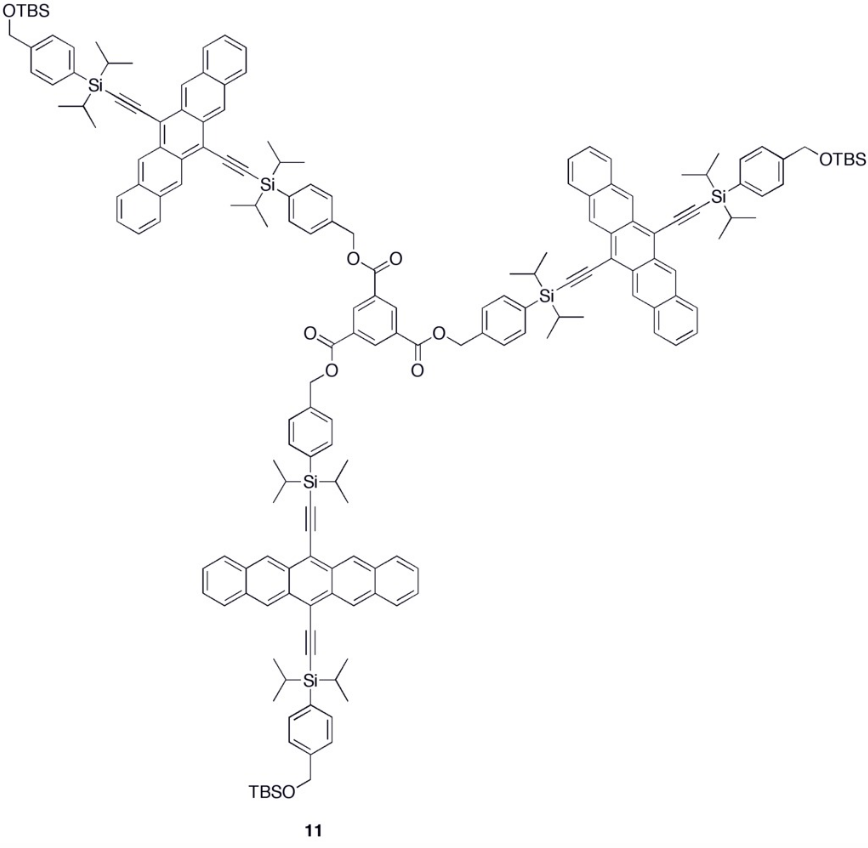
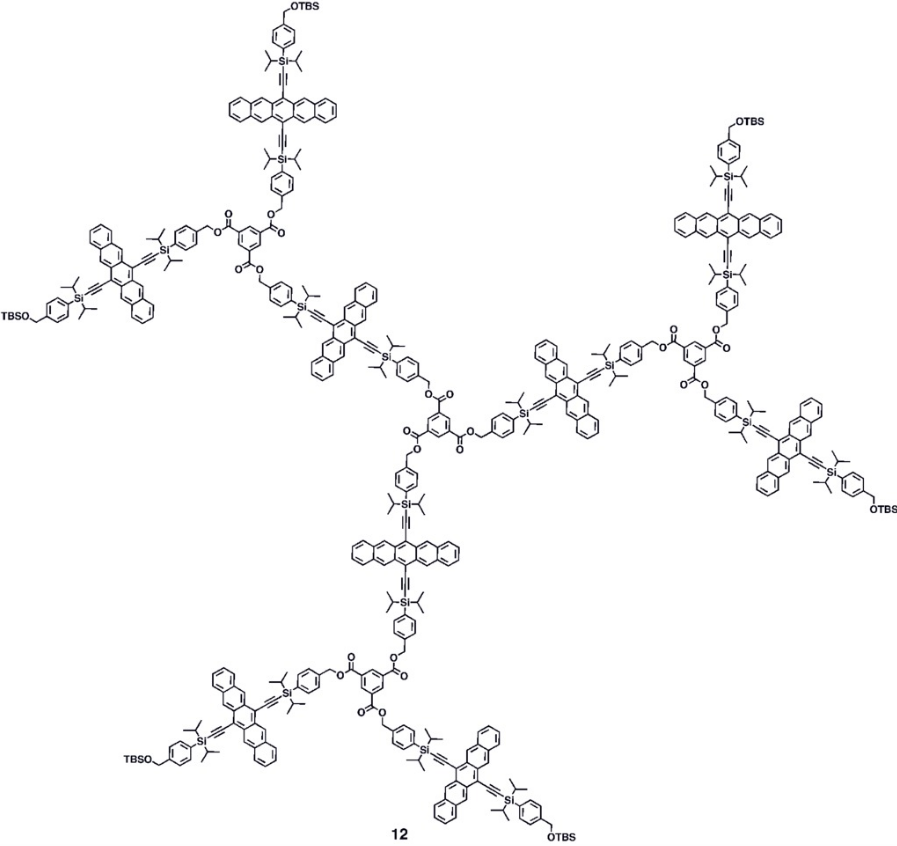
Various synthetic strategies have been employed to form conjugated oligomers of pentacene 5a–c including a one-pot-four-bond forming procedure which provided a solution-processable conjugated pentacene dimer (5c) which exhibited photoconductive gain >10,[27] placing its performance within the same order of magnitude as thermally evaporated films of non-functionalized pentacene which exhibited photoconductive gain >16 using analogous measurement techniques.[28] A modular synthetic method to conjugated pentacene di-, tri- and tetramers (6–8) has been reported which is based on homo- and cross-coupling reactions of robust dehydropentacene intermediates.[29] Non-conjugated oligomers 9–10 based on pentacene have been synthesized,[25][26] including dendrimers 9–10 with up to 9 pentacene moieties per molecule with molar absorptivity for the most intense absorption > 2,000,000 M−1•cm−1. Dendrimers 11–12 were shown to have improved performance in devices compared to analogous pentacene-based polymers 4a–b in the context of photodetectors.[30]
By P orbital - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=16259346
3. Materials Research
Pentacenes have been examined as potential dichroic dyes. The pentacenoquinone displayed below is fluorescent and when mixed with liquid crystal E7 mixture a dichroic ratio of 8 is reached.[31][32] Longer acenes align better in the nematic liquid crystal phase.
Fluorescent acenequinones. By P orbital - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=16259262
Combined with buckminsterfullerene, pentacene is used in the development of organic photovoltaic prototypes.[33][34] Organic photovoltaic cells are cheaper and more flexible than traditional inorganic cells, which could potentially open doors to solar cells in new markets.[35]
Pentacene is a popular choice for research on organic thin-film transistors and OFETs, being one of the most thoroughly investigated conjugated organic molecules with a high application potential due to a hole mobility in OFETs of up to 5.5 cm2/(V·s), which exceeds that of amorphous silicon.[36][37][38]
Pentacene, as well as other organic conductors, is subject to rapid oxidation in air, which precludes commercialization. If the pentacene is preoxidized, the pentacene-quinone is a potential gate insulator, then the mobility can approach that of rubrene – the highest-mobility organic semiconductor – namely, 40 cm2/(V·s). This pentacene oxidation technique is akin to the silicon oxidation used in the silicon electronics.[37]
The content is sourced from: https://handwiki.org/wiki/Chemistry:Pentacene
References
- Dinca, L. E.; De Marchi, F.; MacLeod, J. M.; Lipton-Duffin, J.; Gatti, R.; Ma, D.; Perepichka, D. F.; Rosei, F. (2015). "Pentacene on Ni(111): Room-temperature molecular packing and temperature-activated conversion to graphene". Nanoscale 7 (7): 3263–3269. doi:10.1039/C4NR07057G. PMID 25619890. Bibcode: 2015Nanos...7.3263D. https://dx.doi.org/10.1039%2FC4NR07057G
- Mills, William Hobson; Mills, Mildred (1912). "CCXXX.—The synthetical production of derivatives of dinaphthanthracene". J. Chem. Soc., Trans. 101: 2194–2208. doi:10.1039/CT9120102194. https://zenodo.org/record/2012649.
- Chung, Hyunjoong; Diao, Ying (2016). "Polymorphism as an emerging design strategy for high performance organic electronics". J. Mater. Chem. C 4 (18): 3915–3933. doi:10.1039/C5TC04390E. "Since its synthesis in 1912 to the categorization of at least four different polymorphs in 2003, pentacene has developed into a benchmark organic semiconductor due to its excellent thin film transistor performance". https://dx.doi.org/10.1039%2FC5TC04390E
- Elbs, Karl (1886). "Beiträge zur Kenntniss aromatischer Ketone. Erste Mittheilung" (in de). J. Prakt. Chem. 33 (1): 180–188. doi:10.1002/prac.18860330119. https://zenodo.org/record/1427908.
- Breitmaier, Eberhard; Jung, Günther (2005). "12.5.3 Elbs-Reaktion" (in de). Organische Chemie: Grundlagen, Stoffklassen, Reaktionen, Konzepte, Molekülstrukturen (5th ed.). Stuttgart: Georg Thieme Verlag. p. 183. ISBN 9783135415055. https://books.google.com/books?id=Ld-AGnffxXIC&pg=PA183.
- Chen, Kew-Yu; Hsieh, Hsing-Hung; Wu, Chung-Chih; Hwang, Jiunn-Jye; Chow, Tahsin J. (2007). "A new type of soluble pentacene precursor for organic thin-film transistors". Chem. Commun. 2007 (10): 1065–1067. doi:10.1039/b616511g. PMID 17325807. http://ntur.lib.ntu.edu.tw//bitstream/246246/148626/1/64.pdf.
- Allen, C. F. H.; Bell, Alan (1942). "Action of Grignard Reagents on Certain Pentacenequinones, 6,13-Diphenylpentacene". Journal of the American Chemical Society 64 (6): 1253–1260. doi:10.1021/ja01258a005. https://dx.doi.org/10.1021%2Fja01258a005
- Maulding, D. R.; Roberts, Bernard G. (1969). "Electronic absorption and fluorescence of phenylethynyl-substituted acenes". The Journal of Organic Chemistry 34 (6): 1734–1736. doi:10.1021/jo01258a045. https://dx.doi.org/10.1021%2Fjo01258a045
- Li, Shi; Zhou, Lishan; Nakajima, Kiyohiko; Kanno, Ken-Ichiro; Takahashi, Tamotsu (2010). "Synthesis of 1,2,3,4,8,9,10,11-Octasubstituted Pentacenequinone Derivatives and their Conversion into Substituted Pentacenes". Chemistry: An Asian Journal 5 (7): 1620–6. doi:10.1002/asia.200900754. PMID 20455241. https://dx.doi.org/10.1002%2Fasia.200900754
- Takahashi, Tamotsu; Kitamura, Masanori; Shen, Baojian; Nakajima, Kiyohiko (2000). "Straightforward Method for Synthesis of Highly Alkyl-Substituted Naphthacene and Pentacene Derivatives by Homologation". Journal of the American Chemical Society 122 (51): 12876–12877. doi:10.1021/ja003130g. https://dx.doi.org/10.1021%2Fja003130g
- Takahashi, Tamotsu; Li, Shi; Huang, Wenying; Kong, Fanzhi; Nakajima, Kiyohiko; Shen, Baojian; Ohe, Takahiro; Kanno, Ken-Ichiro (2006). "Homologation Method for Preparation of Substituted Pentacenes and Naphthacenes". The Journal of Organic Chemistry 71 (21): 7967–77. doi:10.1021/jo060923y. PMID 17025283. https://dx.doi.org/10.1021%2Fjo060923y
- Takahashi, Tamotsu; Li, Yanzhong; Hu, Jinghan; Kong, Fanzhi; Nakajima, Kiyohiko; Zhou, Lishan; Kanno, Ken-Ichiro (2007). "Cu(I)-mediated cycloaddition reaction of zirconacyclopentadienes with fumaronitrile and application for synthesis of monocyano-substituted pentacenes". Tetrahedron Letters 48 (38): 6726–6730. doi:10.1016/j.tetlet.2007.07.075. https://dx.doi.org/10.1016%2Fj.tetlet.2007.07.075
- Stone, Matthew T.; Anderson, Harry L. (2007). "Three-Step Synthesis of End-Substituted Pentacenes". The Journal of Organic Chemistry 72 (25): 9776–8. doi:10.1021/jo7017284. PMID 17999529. https://dx.doi.org/10.1021%2Fjo7017284
- Li, Shi; Li, Zhiping; Nakajima, Kiyohiko; Kanno, Ken-Ichiro; Takahashi, Tamotsu (2009). "Double Homologation Method for Substituted Soluble Pentacenes and Dimerization Behaviours of Pentacenes". Chemistry: An Asian Journal 4 (2): 294–301. doi:10.1002/asia.200800312. PMID 19072938. https://dx.doi.org/10.1002%2Fasia.200800312
- Anthony, J. E.; Brooks, J. S.; Eaton, D. L.; Parkin, S. R. (2001). "Functionalized Pentacene: Improved Electronic Properties from Control of Solid-State Order". Journal of the American Chemical Society 123 (38): 9482–9483. doi:10.1021/ja0162459. PMID 11562247. https://dx.doi.org/10.1021%2Fja0162459
- Anthony, J. E.; Eaton, D. L.; Parkin, S. R. (2002). "A Road Map to Stable, Soluble, Easily Crystallized Pentacene Derivatives". Organic Letters 4 (1): 15–18. doi:10.1021/ol0167356. PMID 11772079. https://dx.doi.org/10.1021%2Fol0167356
- Norton, Joseph E.; Northrop, BH; Nuckolls, C; Houk, KN (2006). "Why 6-Methylpentacene Deconjugates but Avoids the Thermally Allowed Unimolecular Mechanism". Organic Letters 8 (21): 4915–8. doi:10.1021/ol062012g. PMID 17020335. https://dx.doi.org/10.1021%2Fol062012g
- Briseno, Alejandro L.; Miao, Q; Ling, MM; Reese, C; Meng, H; Bao, Z; Wudl, F (2006). "Hexathiapentacene: Structure, Molecular Packing, and Thin-Film Transistors". Journal of the American Chemical Society 128 (49): 15576–7. doi:10.1021/ja066088j. PMID 17147352. . https://dx.doi.org/10.1021%2Fja066088j
- Lu, Jun; Ho, DM; Vogelaar, NJ; Kraml, CM; Bernhard, S; Byrne, N; Kim, LR; Pascal Jr, RA (2006). "Synthesis, Structure, and Resolution of Exceptionally Twisted Pentacenes". Journal of the American Chemical Society 128 (51): 17043–50. doi:10.1021/ja065935f. PMID 17177456. https://dx.doi.org/10.1021%2Fja065935f
- Lehnherr, D.; Tykwinski, R.R. (2010). "Oligomers and Polymers Based on Pentacene Building Blocks". Materials 3 (4): 2772–2800. doi:10.3390/ma3042772. Bibcode: 2010Mate....3.2772L. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5445842
- Lehnherr, D.; Tykwinski, R. R. (2011). "Conjugated Oligomers and Polymers Based on Anthracene, Tetracene, Pentacene, Naphthodithiophene, and Anthradithiophene Building Blocks". Australian Journal of Chemistry 64 (7): 919–929. doi:10.1071/CH11169. https://dx.doi.org/10.1071%2FCH11169
- Tokito, S.; Weinfurtner, K.-H.; Fujikawa, H.; Tsutsui, T.; Taga, Y. (2001). Kafafi, Zakya H. ed. "Acene containing polyfluorenes for red, green and blue emission in organic light-emitting diodes". Proc. SPIE–Int. Opt. Soc. Eng.. Organic Light-Emitting Materials and Devices IV 4105: 69–74. doi:10.1117/12.416877. Bibcode: 2001SPIE.4105...69T. https://dx.doi.org/10.1117%2F12.416877
- Okamoto, T.; Bao, Z. (2007). "Synthesis of solution-soluble pentacene-containing conjugated copolymers". Journal of the American Chemical Society 129 (34): 10308–10309. doi:10.1021/ja0725403. PMID 17685520. https://dx.doi.org/10.1021%2Fja0725403
- Okamoto, T.; Okamoto, T.; Jiang, Y.; Qu, F.; Mayer, A.C.; Parmer, J.E.; McGehee, M.D.; Bao, Z. (2008). "Synthesis and characterization of pentacene– and anthradithiophene–fluorene conjugated copolymers synthesized by Suzuki reactions". Macromolecules 41 (19): 6977–6980. doi:10.1021/ma800931a. Bibcode: 2008MaMol..41.6977O. https://dx.doi.org/10.1021%2Fma800931a
- Lehnherr, D.; Tykwinski, R. R. (2007). "Pentacene Oligomers and Polymers: Functionalization of Pentacene to Afford Mono-, Di-, Tri-, and Polymeric Materials". Organic Letters 9 (22): 4583–4586. doi:10.1021/ol702094d. PMID 17918951. https://dx.doi.org/10.1021%2Fol702094d
- Lehnherr, Dan; McDonald, Robert; Ferguson, Michael J.; Tykwinski, Rik R. (2008). "Synthesis of soluble oligo- and polymeric pentacene-based materials". Tetrahedron 64 (50): 11449–11461. doi:10.1016/j.tet.2008.09.041. ISSN 0040-4020. https://dx.doi.org/10.1016%2Fj.tet.2008.09.041
- Lehnherr, D.; Gao, J.; Hegmann, F. A.; Tykwinski, R. R. (2008). "Synthesis and Electronic Properties of Conjugated Pentacene Dimers". Organic Letters 10 (21): 4779–4782. doi:10.1021/ol801886h. PMID 18823120. https://dx.doi.org/10.1021%2Fol801886h
- Gao, J.; Hegmann, F. A (2008). "Bulk photoconductive gain in pentacene thin films". Applied Physics Letters 93 (22): 223306. doi:10.1063/1.3043431. Bibcode: 2008ApPhL..93v3306G. https://dx.doi.org/10.1063%2F1.3043431
- Lehnherr, D.; Murray, A. H.; McDonald, R.; Tykwinski, R.R. (2010). "A Modular Synthetic Approach to Conjugated Pentacene Di-, Tri-, and Tetramers". Angewandte Chemie International Edition 49 (35): 6190–6194. doi:10.1002/anie.201000555. PMID 20645363. https://dx.doi.org/10.1002%2Fanie.201000555
- Lehnherr, D.; Gao, J.; Hegmann, F. A.; Tykwinski, R. R. (2009). "Pentacene-based dendrimers: synthesis and thin film photoconductivity measurements of branched pentacene oligomers". Journal of Organic Chemistry 74 (14): 5017–5024. doi:10.1021/jo9007089. PMID 19489566. https://dx.doi.org/10.1021%2Fjo9007089
- Chen, Zhihua; Swager, TM (2007). "Synthesis and Characterization of Fluorescent Acenequinones as Dyes for Guest−Host Liquid Crystal Displays". Organic Letters 9 (6): 997–1000. doi:10.1021/ol062999m. PMID 17298074. https://dx.doi.org/10.1021%2Fol062999m
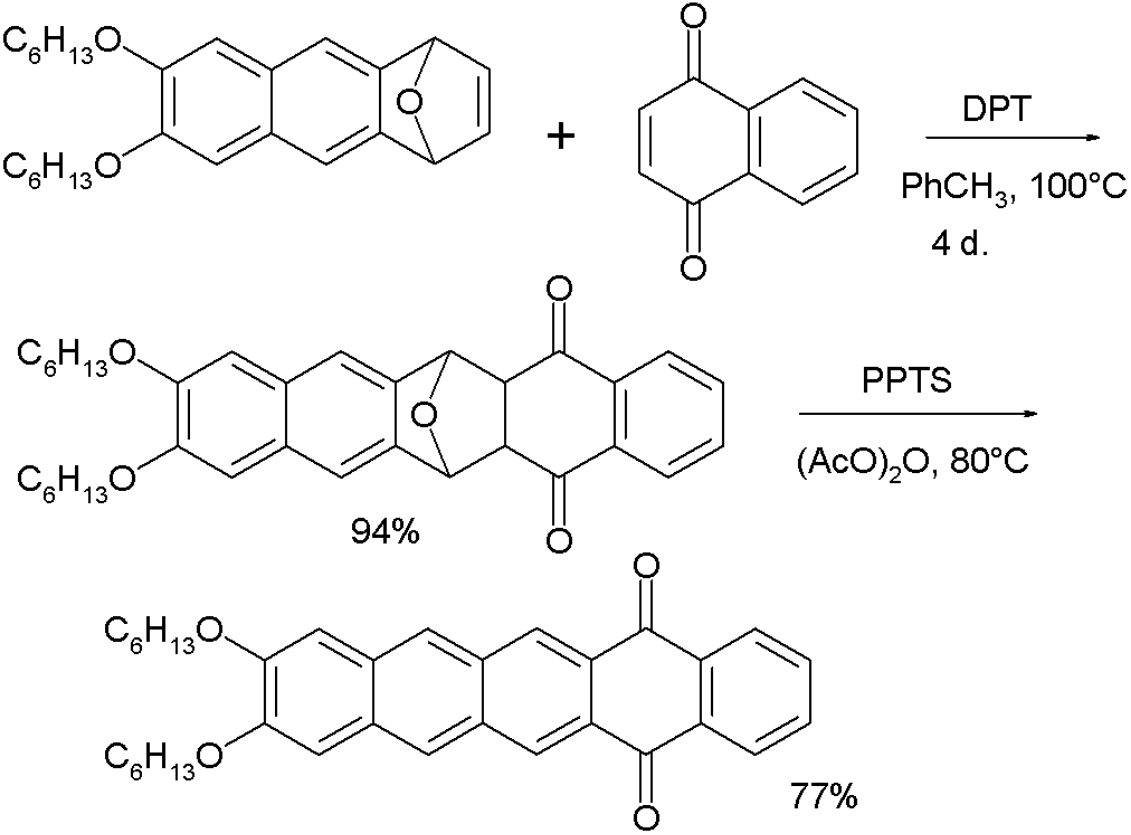
- in the synthesis of this compound, the starting material is treated with 1,4-naphthoquinone and DPT. DTP converts the oxo-norbornadiene to an intermediary furan. The second step is oxidation by PPTS
- Dissanayake, D. M. Nanditha M. (2007). "Nanoimprinted large area heterojunction pentacene-C[sub 60 photovoltaic device"]. Applied Physics Letters 90 (25): 253502. doi:10.1063/1.2749863. Bibcode: 2007ApPhL..90y3502D. http://epubs.surrey.ac.uk/7807/1/fulltext.pdf.
- Efficiently Organic: Researchers Use Pentacene To Develop Next-generation Solar Power sciencedaily.com Link https://www.sciencedaily.com/releases/2004/12/041220005834.htm
- "Efficiently Organic: Researchers Use Pentacene To Develop Next-generation Solar Power" (in en). ScienceDaily. https://www.sciencedaily.com/releases/2004/12/041220005834.htm.
- Norbert Koch (2007). "Organic Electronic Devices and Their Functional Interfaces". ChemPhysChem 8 (10): 1438–55. doi:10.1002/cphc.200700177. PMID 17539032. https://dx.doi.org/10.1002%2Fcphc.200700177
- Tatsuo Hasegawa; Jun Takeya (2009). "Organic field-effect transistors using single crystals". Sci. Technol. Adv. Mater. 10 (2): 024314. doi:10.1088/1468-6996/10/2/024314. PMID 27877287. Bibcode: 2009STAdM..10b4314H. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5090444
- Yoshiro Yamashita (2009). "Organic semiconductors for organic field-effect transistors". Sci. Technol. Adv. Mater. 10 (2): 024313. doi:10.1088/1468-6996/10/2/024313. PMID 27877286. Bibcode: 2009STAdM..10b4313Y. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5090443