Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Analytical
Carbon quantum dots (CQDs) are small carbon NPs with a size less than 10 nm having excellent conductivity, chemical stability, environmental friendliness, high photostability, broadband optical absorption, low toxicity, photobleaching resistance, high surface area, and ease of modification. Graphene quantum dots (GQDs) are two-dimensional nanocrystals composed of small graphene particles with lateral diameters less than 100 nm.
- carbon quantum dots
- graphene quantum dots
- sensors
1. Introduction
Carbon-based materials have gained immense attention for environmental decontamination owing to their appealing physicochemical characteristics viz large specific surface area, tunable structure, low toxicity, and outstanding stability. Quantum dots (QDs) are semiconductor nanoparticles (NPs) of a size less than 10 nm, confined inside spatial dimensions with quantized energy states [1,2]. The atoms in the QDs are arranged similarly to those in bulk materials, however because of 3-dimensional truncation, there are more atoms on their surfaces. QDs have exceptional luminescent and electrical features, including a narrow emission, broad and continuous absorption spectrum, and great light stability [3,4].
Semiconductor-based QDs are highly effective inorganic fluorescent probes, having long-term photobleaching resistance. In terms of biocompatibility, chemical inertness, and low cytotoxicity, carbon-based QDs viz. carbon quantum dots (CQDs) and graphene quantum dots (GQDs) outperform the typical semiconductor QDs such as CdSe dots, ZnO dots and others [5,6,7,8]. They utilize easily available natural resources as raw materials, and have a number of advantages, including wavelength-dependent luminescence emission, ease of synthesis, and bioconjugation [9,10]. Their optical characteristics are improved by the non-zero band gap. They also exhibit edge effects caused by the existence of a functional group on their surface. In the case of carbon-based QDs, a broad photoluminescence emission with strong excitation wavelength dependency and broad absorption bands are seen [11].
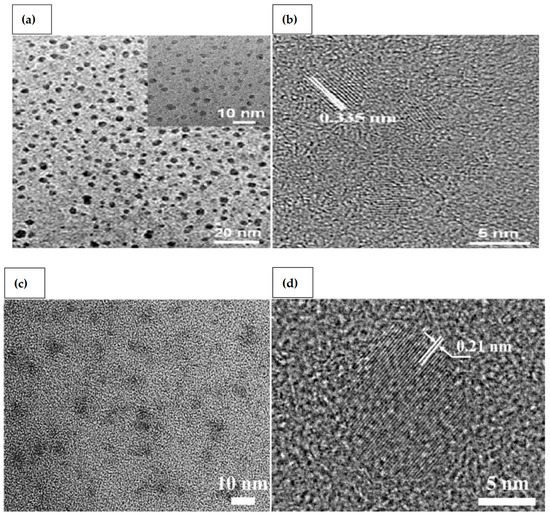
2. CQDs and GQDs
CQDs are small carbon NPs with a size less than 10 nm having excellent conductivity, chemical stability, environmental friendliness, high photostability, broadband optical absorption, low toxicity, photobleaching resistance, high surface area, and ease of modification. The photoluminescent properties of CQDs are their most notable feature [20,21]. CQDs have high fluorescence stability, which indicates that the fluorescence emission intensity can remain constant even after a long time of continuous stimulation. CQDs are an appealing option in sensor applications because of their unique properties [22]. Due to many carboxyl groups on their surface, CQDs have outstanding potential to either contain appropriate chemically reactive groups for functionalization, or to connect with diverse polymeric, organic, biological, inorganics, or natural materials, for surface modification. Surface passivation promotes fluorescence, and surface functionalization improves solubility in both aqueous and non-aqueous media [23]. When there is a lot of oxygen in the air, it creates a new energy state inside the band gap of CQDs, which causes the diameter to shift [24]. Thus, CQDs as sensors have grabbed the interest of researchers [25].
GQDs are two-dimensional nanocrystals composed of small graphene particles with lateral diameters less than 100 nm. Single atomic layered GQDs containing only carbon are ideal. They are also known to include oxygen and hydrogen, as well as many layers having a thickness of less than 10 nm. GQDs have an adjustable band gap energy between 0 and 6 eV. Due to the quantum confinement effect, and conjugated edge effects, the band gap can be tailored by modifying the lateral size or surface chemical characteristics [26]. GQDs have exceptional optoelectronic capabilities, high biocompatibility, and low manufacturing costs, making them a viable alternative to well-known metal chalcogenides-based quantum dots. In addition, compared to typical semiconductor quantum dots, graphene has remarkable thermal and electrical conductivity because of the bonding below and above the atomic plane [27,28]. Tunable photoluminescence (PL), outstanding biocompatibility, remarkable spin property, exceptional UV-blocking capability, and high photo stability, are among the wide range of excellent properties of GQDs, along with other physical characteristics such as high surface area and surface grafting assistance through conjugated network [29]. As the pH of GQDs solution is increased from 1.0 to 11.0, a red shift from 522 to 575 nm is detected [30]. This is caused by deprotonation of oxygen-containing functional groups like carboxyl, hydroxyl, and epoxy. A peak at 626 nm is detected at pH levels of more than 11.0, giving it red florescence. They are quite sensitive to changes in pH and temperature, and even with a small change in temperature or pH, a rapid shift in fluorescence has been reported. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images reveal monodispersed GQDs and CQDs [31,32] (Figure 1). Because of their distinctive photoluminescence properties and tendency to conjugate with various biomolecules, CQDs and GQDs have gained much interest in biomedical and bioanalytical applications. The development of sensitive bioanalysis systems for use in point-of-care testing would also benefit from the usage of CQDs and GQDs in conjunction with their unique optical properties. The simple accessibility of several types of carbon dots for therapeutic usage may mark a discernible improvement in their application. Excellent performance and excellent practicality have been demonstrated for the CQDs-based biomacromolecule detection in point-of-care testing for viruses [33]. Very recently, Li et al. [34] successfully used Gd3+ doped CQDs and specific antibody to detect SARS-CoV-2. Yuan et al. [35] utilized GQDs, gold nanoclusters that were conjugated with antibodies to detect pathogens.

3. Difference between CQDs and GQDs
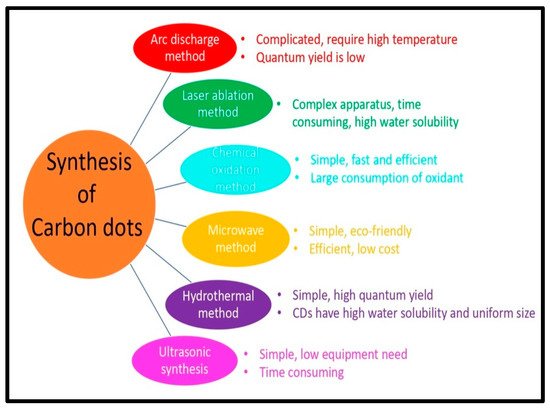
CQDs and GQDs differ in their structural properties. GQDs are crystalline and sp2 hybridized, while CQDs are amorphous and sp3 hybridized. Surface defects produce the fluorescence of CQDs, which have a diameter of less than 10 nm. Quantum confinement causes the fluorescence of GQDs, which range in size from 2 to 20 nm [36]. GQDs are distinguished from carbon dots (CDs) by the graphene sheets within the dots, which is less than 100 nm in size and just 10 layers thick. CDs are usually carbon nanoparticles that are quasi-spherical in shape [37]. GQDs are ideal spherical nanocrystals of sp2 carbon nanosheets, whereas other types of carbon-based dots, such as CQDs, have a more complex structure with a carbogenic core, which in some cases resembles graphene nanolayers coupled with amorphous carbon. Two types of carbogenic spherical structures: one with a crystalline core holding both sp2 and sp3 carbons; and the other with a disordered structural core containing predominantly sp3 carbons, can be present. Bandgap transitions are produced by conjugated domains of QDs, which resemble massive aromatic systems with substantial conjugation of certain electronic energy bandgaps for photoluminescence emissions. Such electronic transitions are caused by the absorption of light by a number of high-density electrons in the sp2 hybridized orbitals, which have a substantial absorption band in the ultraviolet region [38]. The synthesis of CQDs, GQDs, and their nanocomposites, is covered in the next two parts. Figure 2 shows different methods of synthesis of CQDs and GQDs.

Figure 2. Different methods for synthesis of CQDs and GQDs and their distinctive features.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors10090367
This entry is offline, you can click here to edit this entry!
