Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
The stubborn and complex structure of lignocellulose hinders the valorization of each component of cellulose, hemicellulose, and lignin in the biorefinery industries. Therefore, efficient pretreatment is an essential and prerequisite step for lignocellulose biorefinery. A considerable number of studies have focused on peroxyacetic acid (PAA) pretreatment in lignocellulose fractionation and some breakthroughs have been achieved in recent decades.
- lignocellulose
- peroxyacetic acid (PAA) pretreatment
- mass balance
1. Introduction
Due to serious environmental issues and global climate change, researchers all over the world are trying their best to convert the fossil fuel-based society into a bio-economical society, advancing the goal of reaching peak carbon and realizing carbon neutrality [1,2]. Although fossil fuels play a critical role in social industrialization, these non-renewable and unsustainable fuels have negative effects on the environment and humans [3,4]. Lignocellulose, such as forest residues (branches, leaves, etc.), agricultural residues (wheat straw, rice straw, etc.), energy crops (willow, poplar, etc.), and cellulosic waste (e.g., municipal solid waste and food waste) are abundant and cost-effective renewable resources with an annual production of 15–17 × 1010 Mt [5,6]. Lignocellulose can be upgraded into biofuels, biochemicals, and biomaterials [7,8]. Therefore, lignocellulose biorefinery is expected to replace the traditional petroleum refining, and this will mitigate energy crisis and environmental pollution [9]. The United Nations Conference on Environment and Development (UNCED) predicts that the utilization of biomass resources may reach half of the world’s total resource use by 2050 [10].
However, pretreatment processes are required to destroy the stubborn structure of lignin, resulting in the improvement of the accessibility of cellulase to cellulose for the downstream utilization [11]. At present, four major methods of lignocellulose pretreatment are described in the literature [12]. Each method has its own advantages and disadvantages. For instance, physical pretreatment, such as milling and grinding, can improve the surface area and porosity of lignocellulose, but the high energy consumption of this pretreatment increases the operational costs and limits its practical applications [13]. Chemical pretreatment of dilute acids, bases, organic solvents, ionic liquids, and low eutectic solvents can remove lignin and hemicellulose to improve the enzymatic accessibility of cellulose, and can also reduce the degree of polymerization (DP) and crystallinity (Crl) of cellulose [14]. However, a critical issue in chemical pretreatment is that chemical reagents are expensive and prone to corrode equipment. Physico-chemical pretreatment is a combination of physical and chemical pretreatment; this method can dissolve lignin and hemicellulose to facilitate the utilization of cellulose [15]. Typical physicochemical pretreatment includes steam explosion, liquid hot water, ammonia fiber explosion, ammonia cycle permeation, electrocatalysis, CO2 explosion, and SO2 explosion [16]. The drawbacks of physicochemical pretreatment are that it requires high temperatures and high-pressure reaction conditions. Biological pretreatment uses microbial communities such as fungi or bacteria to damage the lignocellulosic structure. It is a novel pretreatment method with low energy consumption and low environmental impact [17]. However, an unsatisfactory aspect is that the low efficiency of biodegradation pretreatment limits its large-scale industrial applications [18].
Peroxyacetic acid (PAA), an organic peroxy acid, has been extensively regarded as a disinfectant, strong oxidizer, preservative, bactericide, and polymerization catalyst [19]. In recent years, PAA has been employed as a strong oxidant to oxidize the hydroxyl group in the lignin side chain to the carbonyl group, and it will cleave the β-aryl bond of lignin to reduce the molecular weight and introduce hydrophilic groups [20]. PAA will also oxidize the hydroxyl group in the lignin side chain to hydroquinone; it is subsequently oxidized to quinone, whose ring opening generates water-soluble hydroponic acid, maleic acid, and fumaric acid derivatives [20]. Through these reactions, lignin is depolymerized and the fragments will dissolve in water, leading to effective removal from lignocellulosic biomass [21]. In addition, the oxidized lignin shows low hydrophobicity and weakens the ability to bind to cellulase. Therefore, an increasing number of studies have been focusing on PAA pretreatment in lignocellulosic biorefinery.
2. Lignocellulose Structure
Lignocellulose biomass is an abundant, diverse, and inexpensive renewable resource in nature. It has been universally converted into biofuels, biochemicals, and biomaterials [22]. As shown in Figure 1, lignocellulose is mainly composed of cellulose (40–45%), hemicellulose (20–40%), and lignin (10–25%), which are tightly bound together to form the skeletal framework of plant. The three-dimensional network structure shows that cellulose and hemicellulose are mainly connected by hydrogen bonds, and lignin and hemicellulose are also linked with chemical bonds, such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions [23].
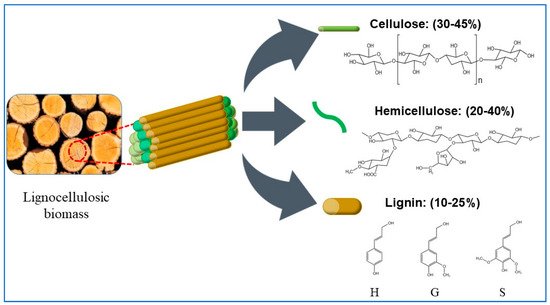
Figure 1. Structural compositions of lignocellulosic biomass.
2.1. Cellulose
Cellulose, the most abundant polymer on Earth, is a linear intercalation (alternating spatial arrangement of side chains) homopolymer. It consists mainly of β-(l-4) glycosidic bonds linked by alternating arrangements [24]. Due to its unique structure of ordered bundle arrangement and highly crystalline structure, cellulose is very stable in many conditions. Cellulose has good biocompatibility and active hydroxyl groups with an atomic O/C of 0.6–0.83 and H/C of 0.8–1.67 [25]. Cellulose can be valorized into fermented glucose [26], bioethanol [27], biomaterials [28,29], and catalyst carrier [30].
2.2. Hemicellulose
Hemicellulose has a heteropolymer with a relatively lower molecular weight compared to cellulose; it is composed mainly of pentoses (e.g., xylose and arabinose) and hexoses (e.g., mannose, glucose, and galactose) [31]. Hemicellulose is bound to various other cell wall components such as fibronectin, cell wall proteins, lignin, and phenolic compounds through covalent bonds, hydrogen bonds, and hydrophobic interactions [32]. Hemicellulose has been mainly used to produce fructose and xylitol. Apart from these products, hemicellulose can also be converted to biofuels [33], furfural [34], levulinic acid; and formic acid [35,36].
2.3. Lignin
Lignin is a polymer of heterogeneous phenyl propane units in plants and consists of three main monomers: guaiacol (G), eugenol (S), and p-hydroxyphenyl (H) [37]. These three monomers are chemically linked with the C-C bond (5-5, β-β, β-1, β-5) and aryl ethers (β-O-4, α-O-4) to yield three corresponding subunits: p-coumaryl alcohol (pCoumA), pineal alcohol (ConA), and mustard alcohol (SinA) [38]. Due to the heterogeneity and complex components, lignin shows strong stubborn and anti-barrier effects [39]. To date, lignin has mainly been used in reinforcing agents [40], binders [41], hydrogels [42], adsorbents [43], and catalysts [44]. Efficient valorization of lignin will be a hot topic of research in the near future.
3. Quick Overview of PAA
3.1. Commercial PAA
Commercial PAA products are greatly dependent on the ratio of PAA to hydrogen peroxide (H2O2). Table 1 provides detailed information on part commercial PAA in the literature. Commercial PAA is usually prepared by mixing H2O2 and acetic acid (or ethyl acetate), catalyzed with concentrated sulfuric acid. The desired concentration and yield of PAA are achieved by adjusting the concentration of H2O2 and the ratio of acetic acid. However, the chemical production of PAA is characterized by flammability, explosiveness, toxicity, high temperature, high pressure, and corrosiveness. From the point-of-view of safety and green chemistry, it is very dangerous to produce commercial PAA in the laboratory.
| Identity | Product Name | Supplier and Country | PAA(%) | H2O2(%) | PAA:H2O2 |
|---|---|---|---|---|---|
| Lspez | Wofasteril L. Spez | KESLA PHARMA WOLFEN GmbH (Greppin, Germany) | 3 | 40 | 0.034 |
| E35 | Wofasteril 035 | KESLA PHARMA WOLFEN GmbH (Greppin, Germany) | 3.5 | 10 | 0.156 |
| SC50 | Wofasteril SC50 | KESLA PHARMA WOLFEN GmbH (Greppin, Germany) | 5 | 8 | 0.28 |
| AC150 | Peressigsaure 15% reinst | Applichem GmbHt (Darmstadt, Germany) | 15 | 24 | 0.28 |
| E250 | Wofasteril E250 | KESLA PHARMA WOLFEN GmbH (Greppin, Germany) | 25 | 30 | 0.37 |
| S1400 | Sigma-Aldrich Peracetic Acid Solution | Sigma-Aldrich Co. (St. Louis, MO, USA) | 39 | 6 | 2.91 |
| E400 | Wofasteril E400 | KESLA PHARMA WOLFEN GmbH (Greppin, Germany) | 40 | 12 | 1.49 |
| S1400 | Sigma-Aldrich 32 wt% PAA | Sigma-Aldrich Co. (St. Louis, MO, USA) | 32 | 5 | 6.4 |
| / | / | Thermo Fisher Scientific (New York, NY, USA) | 39 | / | / |
| VigorOx® WWTII | PAA technical grade solution (VigorOx® WWTII) | PeroxyChem (Philadelphia, Pennsylvania, USA) | 15 | 23 | 0.652 |
3.2. Chemically Activated PAA
To improve the oxidative ability of commercial PAA, some activators can be added to the PAA system. These activators include radiation, metal catalysts, and carbon-based materials [50,51]. For example, the O-O bond in PAA can be directly broken by UV radiation to generate the radicals R-O· and HO·, thus improving disinfection efficiency and the degradation of organic compounds [52]. UV irradiation has been used to activate PAA to form active radicals that degrade naproxen (NAP). This process would be impracticable without sufficient UV intensity, because the penetration of UV light in water is limited [50]. Hu et al. investigated an advanced oxidation technique based on UV/PAA to degrade steroid estrogens Hu, Li, Zhang, et al. [53]. The metal activators of PAA include metal ions (Cu2+, Co2+, Fe2+, and Mn2+) [54,55] and metal oxides (ZVCo, Co2O3, CoFe2O4, and Co3O4) [56]. The mechanism of PAA activation by chemical activators can be triggered through the generation of organic radicals CH3C(O)O· and CH3C(O)OO· (Figure 2); these radicals can degrade organic pollutants by advanced oxidation. Table 2 summarises the degredation of organic pollutants by chemical activation of PAA as reported in the literature.
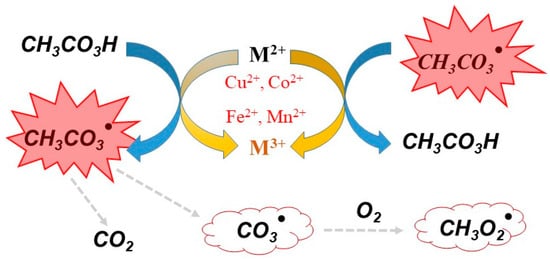
Figure 2. Free radicals generated by PAA in the presence of the metal ions activators (Cu2+, Co2+, Fe2+, and Mn2+).
Table 2. Degradation of organic pollutants by chemical activation PAA.
| Compounds | Chemical Activator (Catalyst) | Compounds Concentration | Conditions: Temperature, pH, Catalyst Loading | PAA Concentration | Degradation Rate (%) | References |
|---|---|---|---|---|---|---|
| Orange G | Co3O4 | 0.05 mM | 25 °C, 7.0, 0.1 g/L | 0.5 mM | 100 | [57] |
| Sulfamethoxazole | CoFe2O4 | 10 μM | 23 °C, 7.0, 0.1 g/L | 100 μM | 87.3 | [56] |
| Bisphenol-A | Co (II)/Co (III) | 15 μM | 22 °C, 4.0, 10 μM, | 100 μM | 100 | [58] |
| Carbamazepine | 87.7 | |||||
| Naproxen | 100 | |||||
| Sulfamethoxazole | 98.5 | |||||
| Sulfamethoxazole | Co | 10 μM | 25 °C, 7.0, 0.8 μM | 100 μM | 89.4 | [51] |
| Naproxen | UV | 4 μM | 20 °C, 7.0, /no catalyst | 20 mg/L | 100 | [50] |
| Bisphenol-A | Fe (II) | 15 μM | 22 °C, 6.0, 5 μM, | 20 μM | 87.7 | [55] |
| Methylene blue | 89.4 | |||||
| Naproxen | 98.2 | |||||
| Sulfamethoxazole | ZVCo * | 5 μM | 25 °C, 7, 0.1 g L−1 | 50 μM | 99.4 | [59] |
| Steroid estrogens | UV | 50 μg/L | 25 °C, 6.01, /no catalyst | 30 mg/L | 90 | [53] |
* ZVCo: zero-valent cobalt.
3.3. Enzymatically Generated PAA
To meet the principle of green chemistry, enzyme-generated PAA has outstanding advantages over commercial and chemically activated PAA. It is a simple, safe, low-cost, and in-situ PAA production method that avoids hazards during storage and transportation [60]. Perhydrolases are critical factors for enzyme-generated PAA, and the most commonly used ones include Pseudomonas fluorescens esterase [20], acetyl xylan esterase [61], and lipase. Perhydrolases can catalyze H2O2 and acetic acid/ethyl acetate for in-situ generation of PAA [49]. Bernhardt et al. reported that the catalytic domain of perhydrolases was Ser-His-Asp Bernhardt, Hult and Kazlauskas [62]. Table 3 summarizes the perhydrolase-producing strains used for enzyme-generated PAA in the literature. Strains-producing perhydrolases are wild microorganisms (Pseudomonas fluorescens, Candida rugosa, Aspergillus niger, Porcine pancreas, Bacillus subtilis CICC 20034, Pichia pastoris) and recombinant strains (Escherichia coli BL21, Aspergillus ficcum). In comparison with commercial PAA, the advantages of enzyme-generated PAA in biomass fractionation are: (1) PAA can be generated as needed, thus eliminating storage-related problems of explosion and stability. (2) Acetyl groups in biomass can be used to generate PAA. (3) PAA will sterilize the biomass to protect it from microbial contamination in biomass storage and fermentation.
Table 3. Perhydrolases producing strains and enzyme-generated PAA.
| Perhydrolase | Strains | Reagent Dosage (EA/GT, H2O2) |
Conditions: Temperature, pH, Enzyme Loading | PAA Concentration (mM) | References |
|---|---|---|---|---|---|
| Pseudomonas fluorescens esterase (PFE) | Pseudomonas fluorescens | 500 mM EA *, 1.0 M H202 | 23 °C, 7.2, 0.5 mg/mL | 115 | [20] |
| Pseudomonas fluorescens esterase (PFE) | Escherichia coli BL21 | 500 mM EA *, 1.0 M H202 |
23 °C, 7.2, 0.5 mg/mL | 90 | [63] |
| PFE-L29G | Pseudomonas fluorescens | 600 mM EA *, 500 mM H202 |
37 °C, 7.0, 0.5 mg/mL | 60 | [49] |
| Wild-type PFE | Pseudomonas fluorescens | 70 | |||
| Lipase Type VII | Candida rugosa | 250 mM GT †, 1.0 M H202 |
25 °C, 7.4, 0.6 mg/mL | 0.98 | [64] |
| LPL | Aspergillus niger | 2.6 | |||
| Lipase Type II | Porcine pancreas | 7.1 | |||
| Acetylxylan esterase (AXE) | Bacillus subtilis CICC 20034 | 113.37 | |||
| Acetylxylan esterase (AXE) | Pichia pastoris | 0.5 M EA *, 1.0 M H202 | 37 °C, 7.0, 15 mg/mL | 133.70 | [65] |
| Recombinant acetylxylan esterase (rAXE) | Aspergillus ficcum | 500 mM EA *, 1.0 M H202 | 37 °C, 7.0, 0.1 mg/mL | 134 | [61] |
* EA: Ethyl acetate; † GT: Glycerol triacetate.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196359
This entry is offline, you can click here to edit this entry!
