Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
One of the most serious complications following the implantation of orthopedic biomaterials is the development of infection. Orthopedic implant-related infections do not only entail clinical problems and patient suffering, but also cause a burden on healthcare care systems.
- orthopedic implants
- bone infections
- bacterial adhesion
- bacteria-material interactions
1. Introduction
In orthopedic surgery and traumatology, bone grafting is one of the most frequently performed surgical procedures used for bone-loss repair and bone augmentation [1]. However, prosthetic joints and other orthopedic implant devices (such as pins, screws, plates, and external fixators), required in situations such as osteomyelitis post-debridement, are extremely sensitive to contamination by microorganisms and the subsequent development of infection [2,3].
Orthopedic implant-related infections are among the main reasons for joint arthroplasty and osteosynthesis failure, with severe and devastating outcomes for patients and health systems. Their treatment requires in most cases, the infected implant removal, implant replacement, revision surgeries, or/and amputation, which translates to high rates of morbidity, and increased risk of mortality [4]. In addition to causing significant physical and emotional suffering, implant infections are a massive economic burden to the health systems, estimated to cost more than $8.6 billion annually in the United States and €2 billion in Europe [4,5]. The implant-related infection rate varies according to the type of bone involved (e.g., hip, knee, ankle, or tibia), grade/type of fracture (i.e., closed or open), or type of surgery (i.e., primary or revision) [6,7,8]. For instance, the likelihood of infection following the implantation of a prosthetic hip is 0.3–2.4%, while a total knee replacement is 1–3% [8]. In closed fractures, the incidence of infection after internal fixation is generally low (0.5–2%) when compared to open fractures, wherein the infection rate may exceed 30% [6,7,8]. In revision surgeries concerning implant removal, amputation, or tissue debridement, the risk of infection is higher when compared to primary ones. For instance, the infection rate following total hip arthroplasties is 14.8%, while for total knee revision it is 25.2% [9].
The Centers for Disease Control (CDC) classified biofilms as one of the most pressing clinical obstacles of the century, since they contribute to more than 80% of human bacterial infections [10,11,12]. Biofilms act as communities of microbial cells attached to an inert or living surface that are functionally organized and enclosed in a self-produced polymeric matrix (EPSs), thus contributing to the increasing infection pathogenicity [13].
2. Implant-Infecting Microorganisms
Implanted materials are highly susceptible to bacterial and fungal colonization and consequent infection [20]. These microorganisms are frequently opportunistic, taking advantage of the weakening of the body defenses at the implant surface–tissue interface, thereby attaching to tissues or implant surfaces, instigating biofilm formation and subsequent development of infection [13,21]. The development of biofilm causes tissue destruction, systemic dissemination of the pathogen and dysfunction of the implant/bone interface, resulting in the failure of implanted material [13,21]. Additionally, the contaminated implant may serve as a reservoir for an infection of the surrounding tissue, where microorganisms may reside intracellularly [13,21].
In Europe and U.S, the most prevalent microorganisms in implant-related infections are Gram-positive bacteria, mainly Staphylococcus aureus (33–43%) and Staphylococcus epidermidis (17–21%) [13,22], as reflected in Table 1. Other Gram-positive bacteria, such as Streptococcus viridans and Enterococcus spp. (mainly Enterococcus faecalis), are encountered in 1–10% and 3–7% of infections, respectively [13,22,23]. Gram-negative organisms, including Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Proteus mirabilis and Proteus vulgaris are less frequent than Gram-positive, causing around 6% of cases [24]. Anaerobic bacteria (including Propionibacterium acnes) and fungi (mainly Candida albicans) are also involved on implant-related infections (2–3%) [13,24]. Polymicrobial infections are reported in about 10–11% of the cases; the majority are caused by two bacterial species such as methicillin-resistant S. aureus (MRSA) and Klebsiella spp. [24,25]. It should be noted that bacteria isolation and identification always depend on the quality of the diagnostic procedure and preceding antimicrobial therapy [24].
Table 1. Prevalence of implant-infecting bacteria in Europe and the U.S. according to the implant type and site.
| Species | Prevalence in Knee Arthroplasty Infections (%) |
Prevalence in Hip Arthroplasty Infections (%) |
Prevalence in Infections Involving External Fixation (%) |
Prevalence in Infections Involving Internal Fixation (%) |
References |
|---|---|---|---|---|---|
| S. aureus | 26.4 | 24.4 | 47.8 | 42.5 | [13,26,27,28] |
| S. epidermidis | 41.8 | 43.6 | 15.2 | 21.9 | [13,26,27] |
| E. faecalis | 2.6 | 3.5 | 8.7 | 5.3 | [13,27] |
| P. aeruginosa | 4.4 | 3.7 | 14.1 | 4.3 | [13,27] |
| E. coli | 5.3 | n/d | n/d | n/d | [13,26,27] |
n/d—not defined.
In the literature, three types of biomaterials-associated infections are reported: (i) exogenous infection that occurs during or immediately after surgery through direct inoculation into the surgical site; (ii) contiguous infections acquired from spread from an adjacent infectious focus, and (iii) hematogenous infections provided from the distant focus of infection, such as blood or lymph. Concerning the time since surgery and the onset of the infection, these infections can be classified into: (i) early infections (less than three months after the surgery); (ii) delayed infections (between three to 24 months after surgery), and (iii) late infections (more than 24 months after surgery) [29]. Early and delayed infections are commonly caused by trauma or contamination during surgery. S. aureus, Enterococcus spp., and Gram-negative bacilli, intrinsically virulent microorganisms, are usually the pathogenic agents related to early infections [13,29]. Coagulase-negative Staphylococci and P. acnes, low-virulent microorganisms, are usually pathogenic agents of delayed infections. Late infections are usually acquired by hematogenous spread, having a considerable range of pathogenic agents originating from the skin, respiratory, dental, or urinary tract infections [13,29,30,31,32].
Methicillin-resistant S. aureus (MRSA), Vancomycin-resistant S. aureus (VRSA), Methicillin-resistant S. epidermidis (MRSE), Vancomycin-resistant Enterococcus (VRE) and extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) are examples of antibiotic-resistant bacteria that have been commonly linked to implant-related infections, which are a huge threat to human health since they limit therapeutic options to adopt [33,34,35]. In addition, these bacteria are producers of virulence factors (e.g., catalase, hyaluronidase, collagenase, toxins) that play an important role in the degree of severity of the infection, once they promote bacterial adherence to the bone and implant and severe tissue damage [13,21].
3. Pathogenesis of Implant-Related Infections
Biofilm formation consists in the irreversible attachment and growth of microorganisms onto surfaces with the concomitant production of the extracellular polymer matrix (EPSs), which alters the microorganism’s phenotypic, growth rate and gene transcription. Biofilms are three-dimensional complex structures that confer significant survival advantages to microorganism communities, which can lead to the recurrence of biofilm-related implant infection. This is a huge concern in the orthopedic field, especially since these communities are highly resilient to host immunity and to conventional anti-microbial therapies [24,36,37,38,39,40,41,42].
Independent of the infection mechanism biofilm formation has four steps (Figure 1): (1) initial adhesion; (2) irreversible adhesion and cell–cell adhesion; (3) proliferation; (4) growth and maturation, and (5) detachment [20,40].
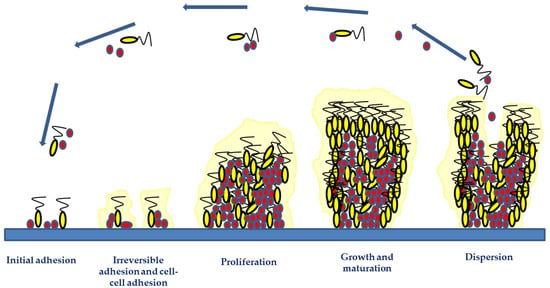
Figure 1. Biofilm development phases include initial adhesion, irreversible adhesion and cell–cell adhesion, proliferation, growth and maturation, and detachment. The two initial phases involve the attachment of microorganisms via hydrophobic or electrostatic interactions to implant surfaces and their involvement in cell-to-cell bindings. The microorganism growth and accumulation during the proliferation and maturation phases result in the development of a mature biofilm structure. Adhesive and disruptive processes occur during the biofilm maturation phase. The final stage of biofilm formation is the detachment phase, involving microbial dispersal and dissemination, which may lead to new infection foci.
The initial microbial attachment consists of the adhesion of planktonic cells to the implant surfaces or the host through hydrophobic or electrostatic interactions between bacteria and surfaces, mediated by: physical shear forces (e.g., van der Waal, steric interactions, and electrostatic forces); microbial appendages (e.g., pili, flagella, or fimbriae), and adhesion surface proteins (e.g., fibronectin, fibrinogen, vitronectin, thrombospondin, laminin, collagen, von Willebrand factor, and polysaccharides) [20,36,40,43]. Twenty surface-associated adhesins are involved in Staphylococcal biofilm formation. These adhesins mediate initial biofilm attachment and intercellular adhesion during maturation-enhancing cohesion. Autolysins (like At1E) mediated the adhesion of S. epidermidis to polymeric surfaces, whereas fibronectin-binding proteins, e.g., FnBPA and FnBPB, induce S. aureus invasion into epithelial cells, endothelial cells, and keratinocytes [20,36,40,42,43].
Biofilm maturation consists of microbial proliferation and aggregation, macro- and microcolonies formation, intercellular signaling and quorum sensing (QS). These are mechanisms that induce the expression of specific genes and proteins involved in biofilm structure, virulence and regulation processes [44]. During biofilm maturation, the microbial cells start the secretion of the EPSs and eDNA, that besides stabilizing the biofilm network into the implant surface, are responsible for linkage between clusters, cell-to-cell cohesion, and cellular communication [44]. The main polysaccharide of S. epidermidis biofilm matrix is polysaccharide intercellular adhesin (PIA), which aids staphylococci in colonizing biomaterial surfaces and protects the proliferating bacteria from polymorphonuclear leukocytes [45]. The microbial microcolonies encased within the EPS communicate between neighboring cells through the QS phenomenon, which controls several physiological processes such as bioluminescence, secretion of virulence factors, biofilm formation and antibiotic resistance [44]. For instance, N-acylated homoserine lactone QS systems of P. aeruginosa are responsible for eDNA release and for biofilm structure [46]. The S. aureus small peptide named AIP QS activates the agrA gene, which regulates the transcription of genes that code for proteases involved in biofilm dispersal [47].
As the biofilm matures, there is an increase in stress-inducing conditions, toxic product accumulation, and limited nutrient availability. These phenomena take the microbial cells to disperse them to other regions of the host’s body or other regions of the medical implant [13,44]. This constitutes the biofilm dispersal phase, where microbial cells (either single cells or clumps of cells) are sloughed off the biofilm. Biofilm dispersal can lead to the dissemination of the detaching microorganisms, which can cause chronic infections, or even reach the bloodstream and cause systemic infections [13,44]. Inhibition of matrix production, enzymatic degradation of EPSs, and surfactant molecules are the mechanisms that contribute to biofilm dispersal. In staphylococci, extracellular enzymes (e.g., staphopain cysteine proteases, the V8 glutamyl endopeptidase SspA and staphylococcal nuclease) and Phenol Soluble Modulins (PSMs) have an essential role in the dispersive phase, particularly in implant-associated biofilm infections. This is because these enzymes are able to degrade the biofilm matrix, contributing to the bacterial dispersal and dissemination of biofilm clusters to distal sites [13,44].
Therefore, developing biomaterials that can prevent bacterial adhesion and/or biofilm formation at the implantation site could be an important breakthrough in bone disease treatments.
This entry is adapted from the peer-reviewed paper 10.3390/ijms231911658
This entry is offline, you can click here to edit this entry!
