Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Drosophila are a well-suited model to unravel the fundamental mechanisms that constitute the innate immune response. NF-κB was originally identified as a DNA-binding activity protein in activated B cells.
- innate immunity
- Drosophila model
- NF-κB pathways
1. Drosophila melanogaster: A Case Study of the Innate Immune System
1.1. Introduction
The immune system is composed of tissues, cells and molecules within an organism that collectively aim to detect agents that are different from the organism’s healthy tissues and organize a response to counteract them and maintain homeostasis. There are two main types of systems: innate and adaptive. The innate immune system precedes the adaptive response and presents conserved mechanisms [1]. It involves a variety of cells and molecular pathways to mount a fast immune response [2][3][4].
The innate immune pathways mainly involve three types of proteins: sensors, which are able to detect microbial patterns or danger signals; adaptors, which are able to transduce the signal downstream of the signaling pathway; and effectors and regulators, which are crucial to the immune response and its dynamicity. When abnormally regulated, innate immune responses contribute to the development of pathologies including chronic inflammation, autoimmune diseases and cancer [5][6]. The notion of intrinsically dynamic regulation proves to be essential in the understanding of the innate immune system.
1.2. Drosophila: An Ever-Relevant Model
Drosophila are a well-suited model to unravel the fundamental mechanisms that constitute the innate immune response. They share many molecular pathways underlying the activation of their innate immune systems with humans [7], and studies have demonstrated the relevance of the model in this context [8]. On a similar note, several studies present Drosophila as a model insect in the field of oncology [9][10][11][12]. In cancer research, flies have been crucial to the discovery of genes and pathways that play oncogenic roles [13][14][15].
The flies present cellular local responses [16] and a systemic immune response. Activation of the second type of response relies on nuclear factor kappa B (NF-κB) pathways. There are two pathways, named immune deficiency (IMD) and Toll, with distinct specificities and characteristics [17][18]. The architecture of the pathways is conserved in mammals, with a strong similarity between IMD and TNFα pathways and between Toll and TLR pathways.
The simplicity of the Drosophila system in respect to mammals allowed the identification or analysis of processes and molecules, as exemplified by the Nobel Prize attributed to Jules A. Hoffmann and Bruce A. Beutler in 2011 for their discovery of the role of Toll receptors in the activation of the innate immune response [19]. It is therefore of great importance to keep an eye on flies’ NF-κB: it is still moving!
2. Overview of the NF-κB Signaling Pathways in Drosophila
NF-κB was originally identified as a DNA-binding activity protein in activated B cells [20]. There are two NF-κB pathways in Drosophila that play a fundamental role in their immune response. The IMD and Toll pathways are able to recognize three main pathogen families: mostly Gram-negative bacteria for IMD, Gram-positive bacteria and fungi for Toll [21].
2.1. The IMD Pathway
Microbial-associated molecular patterns (MAMP) of Gram-negative and some Gram-positive bacteria activate the IMD pathway (Figure 1). Meso-diaminopymelic-type (DAP-type) peptidoglycan is linked to two pattern recognition receptors (PRR) of the peptidoglycan-recognition protein (PGRP) family, PGRP-LC and PGRP-LE [22]. In Drosophila, at least 13 genes encode 17 PGRPs isoforms through alternative splicing [23].
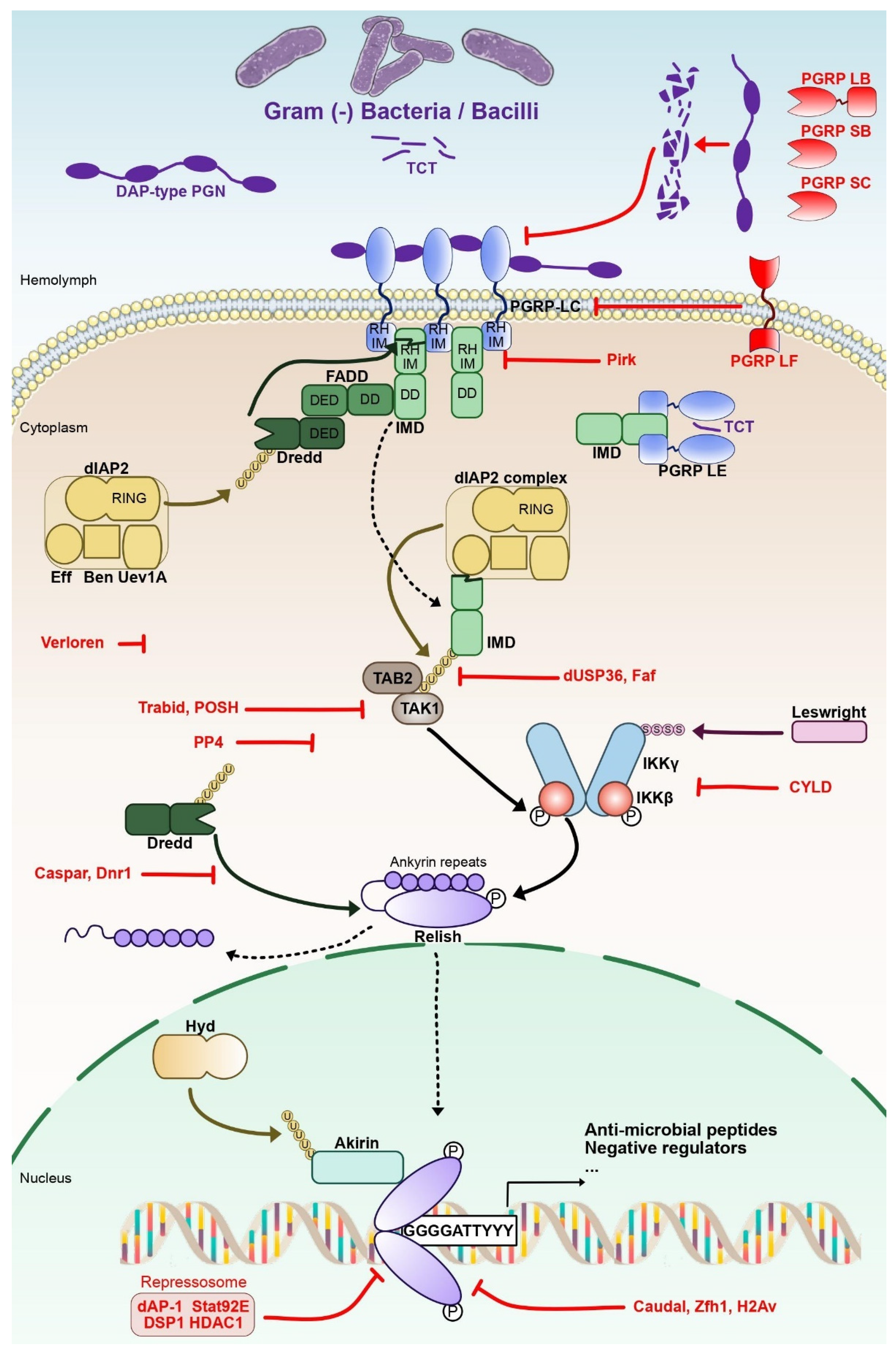
Figure 1. The NF-κB IMD pathway in Drosophila. IMD is activated through the recognition of Gram-negative bacteria-derived meso diaminopymelic-type (DAP-type) peptidoglycan (PGN) and tracheal cytotoxin (TCT) by the Peptidoglycan recognition (PGRP) domain of Peptidoglycan recognition protein -LC and -LE (PGRP LC, -LE). PGRP-LC and -LE death-domains recruit immune deficiency (IMD), FAS-associated death domain (FADD) and death-related ced-3/Nedd2-like protein (Dredd). A ubiquitin-ligase complex formed by the E3 ubiquitin ligase Drosophila inhibitor of apoptosis 2 (DIAP2) and the E2 ubiquitin conjugating Ubiquitin conjugating enzyme variant 1A (Uev1a), Bendless and Effete activates Dredd by K63-linked poly ubiquitinylation. Activated Dredd cleaves IMD N-terminal domain, leading to the recruitment of transforming growth factor beta (TGF-β)-activated kinase 1 (TAK1) and TAK1-associated binding protein 2 (TAB2). TAK1 is able to activate the inhibitor of NF-κB (IκB) kinase (IKK) complex formed of IKKβ and IKKγ subunits. Phosphorylated IKKβ is sumoylated by Leswright and phosphorylates the N-terminal portion of the NF-κB factor Relish to enable its transcriptional activity. Relish is separated from its IκB-like C-terminal ankyrin repeat region by Dredd through proteolytic cleavage. The NLS-containing N-terminal portion of Relish (Rel-68) is then imported to the nucleus while the IκB-like C-terminal portion (Rel-49) remains in the cytoplasm. Rel68 homodimers bind their cognate κB Response element, the consensus sequence 5′-GGGGATTYYY-3′ (Y: C or T) and activate IMD-pathway target genes with the help of the nuclear protein Akirin, which needs to be ubiquitinated by the E3-ligase Hyd beforehand. Dotted arrows indicate the activity of key cleaved proteins of the pathway (IMD, Relish), while continuous arrows are used for the other proteins. Negative regulators are highlighted in red.
The activated receptors PGRP-LC or -LE will interact with the protein adaptor IMD. The cleaved N-terminal of IMD exposes an Inhibitor of Apoptosis 2 (IAP2) binding motif (IBM). That will lead to the recruitment of a tetrameric protein complex composed of DIAP2, Ubiquitin-conjugating variant 1a enzyme (Uev1a), Bendless and Effete complex [24]. The role of this complex is to add Lysine 63 (K63)-linked ubiquitin chains on cleaved IMD, leading to recruitment of the Mitogen-associated protein (MAP) kinase kinase kinase (MAPKKK), Transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) and TAK1-associated binding protein 2 (TAB2) [25]. This complex activates the Inhibitor of NF-κB Kinase (IKK). Composed notably of the catalytic subunit IKKβ (or immune-response deficient 5–Ird5) and the regulatory subunit IKKγ (or Kenny–Key), the IKK complex mediates the phosphorylation and proteolytic cleavage of the NF-κB factor Relish. Relish presents a C-terminal Inhibitor of NF-κB (IκB)-like domain that will remain in the cytoplasm following the cleavage and a N-terminal domain that will translocate from the cytoplasm to the nucleus [26][27][28][29][30].
In the nucleus, Relish proteins form homodimers that control the expression of hundreds of target genes, affecting various immune functions such as microbial recognition, melanization or production of reactive oxygen species [31][32][33]. Some of these genes code for anti-microbial peptides (AMPs), small secreted peptides that play a central role in the defense against micro-organisms [34]. Among the Relish NF-κB target genes, negative regulators are expressed to fine-tune the activation and shutdown of the IMD pathway (Figure 1). Those regulatory proteins can be found at different levels of the pathway: during the DAP-type PGN recognition, at the IMD-IKK signaling platform, for Relish cleavage and activity in the nucleus [35][36][37][38]. For instance, Pickle is a nuclear IκB that interacts with the NF-κB protein Relish, selectively repressing Relish homodimers. Loss of Pickle results in over-expression of Relish target genes. Host resistance to pathogenic bacteria improved in the short term, but chronic inactivation of Pickle shortened the lifespan [39].
2.2. The Toll Pathway
The Toll pathway is activated upon the sensing of fungi, Gram-positive bacteria and some Gram-negative bacteria. The transmembrane receptor Toll is activated by an extracellular proteolytic signaling cascade in two ways: circulating PRR recognizes Lys-type peptidoglycan (Lys-PGN) from Gram-positive bacteria or β-glucans from fungi [40]; proteases produced by fungi, Gram-positive bacteria and some Gram-negative bacteria are sensed by the proteolytically activable serine protease Persephone (Psh), initiating the “danger-signal” pathway [41][42][43].
The extracellular signaling cascade leads to activation of Spätzle (Spz) and its binding to Toll. This initiates the internalization of the receptor and recruitment of the adaptor protein Myeloid differentiation primary response gene 88 (MyD88) through their common TIR domains [44][45][46][47]. MyD88 recruits a secondary adaptor, Tube, through its death domain (DD), leading to the formation of a tripartite complex with Pelle, a kinase homolog to the mammalian Interleukin-1 receptor associated kinase 1 (IRAK1). Pelle phosphorylates the Ankyrin-repeats containing IκB-like protein Cactus [46][47][48][49][50]. The subsequent degradation of Cactus by the proteasome releases the NF-κB factors Dorsal or Dorsal-related immunity factor (DIF). They translocate to the nucleus and exert their DNA-binding activity [51][52][53][54]. Nine Toll-related receptors (Toll-1 to -9) have been identified, with Toll (also called Toll-1) being the main receptor for NF-κB-dependent AMPs synthesis [55]. The principal Toll AMPs are the anti-Gram-positive bacterial Defensin and the antifungal Drosomycins and Metchnikowin [56][57]. Upon activation of both IMD and Toll pathways, formation of heterodimers of Relish and DIF or Dorsal leads to both IMD and Toll pathway target gene expression [58].
Toll pathway activation must also be controlled to prevent putative harmful activations. Only one negative regulator has been identified up until now: Pellino, which regulates Myd88 protein stability [59]. While this work is in contradiction with a previous study that showed Pellino’s requirement for Toll signaling [60], this protein is part of the only feedback regulatory loop described in the Toll pathway that prevents excessive activation. Hoping that further work will help characterize better the regulation of the NF-κB factor DIF activity and the differences with the regulation of Relish.
2.3. Regarding Both Pathways
A difference between the two pathways is the timing of activation: the IMD pathway is rapidly activated within minutes in cell cultures and AMP gene expression culminates around 6 h post stimulation, whereas the Toll pathway takes longer to get activated, with a peak of gene expression around 24 h post stimulation. There is no clue if this is due to differences in the mode of activation of the pathways or something else.
Recent work in various laboratories provides a more comprehensive view of the IMD pathway, its regulation and the dynamic control of gene expression by its NF-κB factor, Relish.
3. Regulation of NF-κB Relish Target Genes Expression
The study of the molecular cascade of the IMD pathway in Drosophila led to the identification of the nuclear protein Akirin by researchers' laboratory. This evolutionarily conserved player in the NF-κB pathway is required for IMD target gene expression by the Relish transcription factor (Figure 2). Its knock-down in flies leads to a high susceptibility to infections due to the lack of expression of most AMPs [61].
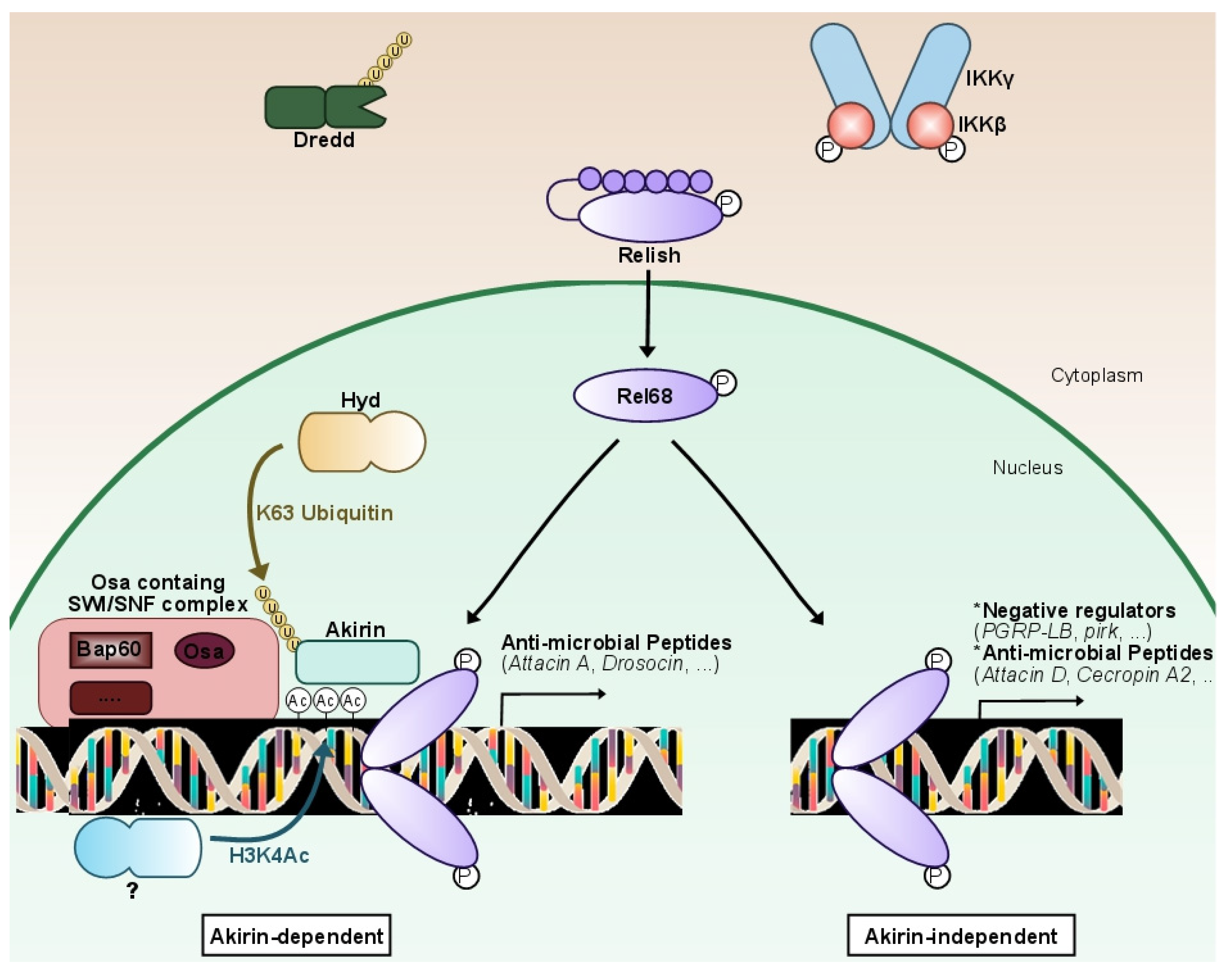
Figure 2. Fine-tuning characterizes NF-κB IMD pathway expression. Following the stimulation of the IMD pathway, a non-identified epigenetic-related protein will deposit an acetyl group on the lysine 4 of histone 3 (H3K4Ac), nearby genes mostly coding for effectors (anti-microbial peptides). After being K63 ubiquitinilated by the E3-ligase Hyd, the conserved nuclear protein Akirin orchestrates a NF-κB transcriptional selectivity through the recruitment of the Osa-containing-SWI/SNF-like Brahma complex (BAP). The N-terminal portion of Relish (Rel-68) will then be recruited to the Akirin complex formed, which will lead to the expression of mostly effector genes of the pathway (anti-microbial peptides). In the case that the H3K4Ac mark is not deposited, Akirin will not be recruited. Rel68 will still bind to the consensus sequence and activate the expression of a second subset of genes, comprised of mostly negative regulators and some anti-microbial peptides. In brief, Akirin is a NF-κB co-factor acting as a molecular selector, required for the activation of a specific subset of Relish-dependent genes that correlates with the presence of H3K4Ac epigenetic marks. Akirin specifies the choice between subsets of NF-κB target genes, allowing Drosophila to modulate its innate immune response.
3.1. First Discovery of Akirin in Innate Immunity
Akirin was identified by a genome-wide RNAi screening as a positive regulator of the IMD pathway [61]. Knock-down of Akirin in Drosophila Schneider 2 (S2) cells reduced the induction of specific IMD pathway regulated antimicrobial peptides, like AttacinA, by 90%. Subsequent epistatic analysis using S2 cells indicated that Akirin acts downstream of or at the level of Relish. Akirin encodes a putative 201 amino acid protein with no recognizable domains but with a clear nuclear localization signal (NLS). This small nuclear protein is highly conserved in metazoan species and consists of one copy in insects and worms and two in vertebrates (except for birds), but none in plants, yeast or bacteria. Complete knockouts of Drosophila Akirin and mouse Akirin 2 led to early embryonic lethality at an early or middle stage, but it was not the case in Akirin1 KO mice [62].
Both Drosophila Akirin and mammalian Akirin2 are required for the innate immune response. Since Akirins have no obvious DNA binding domain, it was proposed that Akirin could be a cofactor that regulates or fine-tunes NF-κB transcriptional activity by interacting with chromatin remodeling factors and/or the transcriptional machine [61][62][63].
3.2. Akirin Fine-Tune the NF-κB Response in Drosophila and Mammals
After stimulation, Akirin is K63-polyubiquitinalyted through the activity of the Hyd E3 ubiquitin ligase [64]. This leads to its binding to Relish, but how this ubiquitination is triggered is unknown. An interesting feature is that Akirin is only required for the activation of a subset of Relish target genes [65]. Indeed, most of Relish-regulated AMP genes are dependent on Akirin, whereas most regulators (including Pirk, PGRP-LB and PGRP-LF) are only dependent on Relish. Some AMP genes are, however, independent of Akirin. Researchers have shown that Akirin-independent gene expression is detected as soon as one hour post-stimulation in cell culture, whereas Akirin-dependent gene expression is only detected at two to three hours.
One interesting hypothesis would be that a moderate or short-time induction of the pathway would only activate, in an Akirin-independent process, a few AMPs to fight infection and most of the regulators of the pathway to maintain the homeostatic state, whereas a strong or prolonged infection would lead to the full Akirin-dependent activation of the pathway, efficient immune response and its subsequent resolution. This would protect against unnecessary activation by commensal or weak infections easily handled by epithelial and phagocytic immune responses. In this model, Akirin-independent genes would be easier and therefore quicker to activate than Akirin-dependent genes. This points to a different epigenetic state of these two classes of genes and a specific function of Akirin to allow activation of less prone-to-activation genes.
As in Drosophila, mammalian Akirin-2 acts downstream of the TLR, TNFR and IL-1R signaling pathways [62]. A conditional knockout of akirin-2 in macrophages compromised the immune response of mice against Listeria monocytogenes intra-peritoneal infections in vivo. Interestingly, mAkirin-2 is required for the regulation of only a subset of LPS and IL-1 inducible genes with mainly pro-inflammatory activity. Moreover, like in Drosophila, mAkirin-2 bridges the NF-κB factor and the chromatin remodeling SWI/SNF complex. It also appeared to participate in the innate immune response through its interaction with the nuclear IκB protein IκBζ, an atypical member of the IκB protein family [62].
It was suggested that IκBζ may influence the regulation of histone modification through selective H3K4 tri-methylation of TLR-induced promoters [66]. An increasing number of studies report that IκBζ regulates the activity of the canonical NF-κB p50 transcription [67][68][69]. As there is no homolog of IκBζ in Drosophila, another cofactor could be implicated and remains to be identified. Other studies show a NF-κB-dependent immune function of Akirins against Gram-negative bacterial infections or more generally, an indispensable role for the expression of innate immune defense genes [70][71]. These results argue for a conserved role of Akirins to partly regulate the innate immune response of metazoans.
3.3. Mechanism of NF-κB Selective Response
Devoid of known predicted functional domains in their sequence, Akirins might gather chromatin-remodeling complexes with sequence-specific targeting transcription factors [72] (Figure 2). Using a genome-wide approach, researchers' laboratory showed that the conserved nuclear protein Akirin is a NF-κB co-factor required for the activation of a subset of Relish-dependent genes, characterized by the presence of the H3K4ac epigenetic mark [65]. This mark is not present on the promoters of the other genes activated by Relish. A large-scale unbiased proteomic analysis revealed that Akirin orchestrates NF-κB transcriptional selectivity through the recruitment of the Brahma-associated protein (BAP) SWI/SNF chromatin-remodeling complex. These findings, conserved from Drosophila [65] to mammals [62], link chromatin remodeling to epigenetic control of NF-κB target gene selectivity. Removing Akirin or SWI/SNF leads to an impaired expression of several AMP-coding genes, affecting the innate immune response of Drosophila against Gram-negative bacteria and worsening survival after infection [65].
Another group also found a diminution of IMD pathway activation after inhibition of BAP complex genes in cells but, surprisingly, an increase in several IMD genes in vivo [73]. Researchers cannot explain this discrepancy at the moment. Moreover, Akirin has also been shown to bridge the SWI/SNF chromatin remodeling complex to target genes during muscle development, where Akirin is also necessary for the function of the transcription factor Twist [74]. Several epigenetic-related proteins have been identified to physically interact with core members of the IMD pathway [75]. Among them, DMAP1, a member of the Tip60-p400 histone acetyltransferase complex, is necessary for optimal expression of IMD target genes and physically interacts with Relish and with components of the BAP SWI/SNF remodeling complex [76]. Its relation to Akirin is under investigation.
Another insight into the pivotal function of chromatin regulation during inflammation lies in the role of a distinct SWI/SNF chromatin remodeling complex, the Poly-Bromo associated Brahma (PBAP) complex, as a negative regulator of IMD signaling in the gut [77]. Bap 180, a component specific of the PBAP complex, was shown to directly interact with the IMD transcription factor Relish and to be recruited to the promoter regions of antimicrobial peptides regulated by the IMD pathway. Flies mutant for Bap180 show increased susceptibility to infections by Gram-negative bacteria as a result of elevated expression of pro-inflammatory IMD-target genes/anti-microbial peptides in the gut rather than elevated bacterial load [77].
Whether other epigenetic marks, in addition to H3K4ac, contribute to coordinate Drosophila innate immune response is still a pending question. Moreover, the precise histone-modifying enzymes involved in the deposition of epigenetic marks in Drosophila remain to be identified. Similarly, whether other chromatin remodeling complexes, in addition to the SWI/SNF complexes, participate in a chromatin remodeling ballet to regulate the expression of immune genes in a dynamic manner remains to be explored.
Of note, mAkirin2 has recently been shown to be required for nuclear entry of proteasomes and turnover of some nuclear factors to control their short-lived activity [78]. Whether this function is independent of its chromatin regulation function remains to be explored.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092304
References
- Dzik, J.M. The ancestry and cumulative evolution of immune reactions. Acta Biochim. Pol. 2010, 57, 443–466.
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852.
- Karin, M.; Lawrence, T.; Nizet, V. Innate Immunity Gone Awry: Linking Microbial Infections to Chronic Inflammation and Cancer. Cell 2006, 124, 823–835.
- Maeda, S.; Omata, M. Inflammation and cancer: Role of nuclear factor-kappaB activation. Cancer Sci. 2008, 99, 836–842.
- Hoffmann, J.A.; Reichhart, J.-M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126.
- Leulier, F.; Lemaitre, B. Toll-like receptors—Taking an evolutionary approach. Nat. Rev. Genet. 2008, 9, 165–178.
- Gonzalez, C. Drosophila melanogaster: A model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 2013, 13, 172–183.
- Villegas, S.N. One hundred years of Drosophila cancer research: No longer in solitude. Dis. Models Mech. 2019, 12, dmm039032.
- Sonoshita, M.; Cagan, R.L. Modeling human cancers in Drosophila. In Current Topics in Developmental Biology; Pick, L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 287–309.
- Enomoto, M.; Siow, C.; Igaki, T. Drosophila as a cancer model. In Drosophila Models for Human Diseases; Yamaguchi, M., Ed.; Springer: Singapore, 2018; pp. 173–194.
- Brumby, A.M.; Richardson, H.E. Using Drosophila melanogaster to map human cancer pathways. Nat. Rev. Cancer 2005, 5, 626–639.
- Parvy, J.-P.; Hodgson, J.A.; Cordero, J.B. Drosophila as a Model System to Study Nonautonomous Mechanisms Affecting Tumour Growth and Cell Death. Bio. Med Res. Int. 2018, 2018, 7152962.
- Gong, S.; Zhang, Y.; Tian, A.; Deng, W.-M. Tumor models in various Drosophila tissues. WIREs Mech. Dis. 2021, 13, e1525.
- Williams, M.J. Drosophila Hemopoiesis and Cellular Immunity. J. Immunol. 2007, 178, 4711.
- Leclerc, V.; Reichhart, J.-M. The immune response of Drosophila melanogaster. Immunol. Rev. 2004, 198, 59–71.
- Imler, J.-L. Overview of Drosophila immunity: A historical perspective. Dev. Comp. Immunol. 2014, 42, 3–15.
- Volchenkov, R.; Sprater, F.; Vogelsang, P.; Appel, S. The 2011 Nobel Prize in Physiology or Medicine. Scand. J. Immunol. 2012, 75, 1–4.
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716.
- Hetru, C.; Hoffmann, J.A. NF-κB in the Immune Response of Drosophila. Cold Spring Harb. Perspect. Biol. 2009, 1, a000232.
- Neyen, C.; Poidevin, M.; Roussel, A.; Lemaitre, B. Tissue- and Ligand-Specific Sensing of Gram-Negative Infection in Drosophila by PGRP-LC Isoforms and PGRP-LE. J. Immunol. 2012, 189, 1886.
- Werner, T.; Liu, G.; Kang, D.; Ekengren, S.; Steiner, H.; Hultmark, D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13772.
- Zhou, R.; Silverman, N.; Hong, M.; Liao, D.S.; Chung, Y.; Chen, Z.J.; Maniatis, T. The Role of Ubiquitination in Drosophila Innate Immunity. J. Biol. Chem. 2005, 280, 34048–34055.
- Kleino, A.; Valanne, S.; Ulvila, J.; Kallio, J.; Myllymäki, H.; Enwald, H.; Stöven, S.; Poidevin, M.; Ueda, R.; Hultmark, D.; et al. Inhibitor of apoptosis 2 and TAK1 binding protein are components of the Drosophila Imd pathway. EMBO J. 2005, 24, 3423.
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226.
- Silverman, N.; Zhou, R.; Erlich, R.L.; Hunter, M.; Bernstein, E.; Schneider, D.; Maniatis, T. Immune Activation of NF-κB and JNK Requires Drosophila TAK1. J. Biol. Chem. 2003, 278, 48928–48934.
- Vidal, S.; Khush, R.S.; Leulier, F.; Tzou, P.; Nakamura, M.; Lemaitre, B. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 2001, 15, 1900–1912.
- Ertürk-Hasdemir, D.; Broemer, M.; Leulier, F.; Lane, W.S.; Paquette, N.; Hwang, D.; Kim, C.-H.; Stöven, S.; Meier, P.; Silverman, N. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9779.
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680.
- Levy, F.; Rabel, D.; Charlet, M.; Bulet, P.; Hoffmann, J.A.; Ehret-Sabatier, L. Peptidomic and proteomic analyses of the systemic immune response of Drosophila. Biochimie 2004, 86, 607–616.
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd Signaling Pathway. J. Immunol. 2014, 192, 3455.
- Ferrandon, D.; Imler, J.-L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862.
- Toke, O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Pept. Sci. 2005, 80, 717–735.
- Guo, L.; Karpac, J.; Tran, S.L.; Jasper, H. PGRP-SC2 Promotes Gut Immune Homeostasis to Limit Commensal Dysbiosis and Extend Lifespan. Cell 2014, 156, 109–122.
- Lhocine, N.; Ribeiro, P.S.; Buchon, N.; Wepf, A.; Wilson, R.; Tenev, T.; Lemaitre, B.; Gstaiger, M.; Meier, P.; Leulier, F. PIMS Modulates Immune Tolerance by Negatively Regulating Drosophila Innate Immune Signaling. Cell Host Microbe 2008, 4, 147–158.
- Maillet, F.; Bischoff, V.; Vignal, C.; Hoffmann, J.; Royet, J. The Drosophila Peptidogly can Recognition Protein PGRP-LF Blocks PGRP-LC and IMD/JNK Pathway Activation. Cell Host Microbe 2008, 3, 293–303.
- Paredes, J.C.; Welchman, D.P.; Poidevin, M.; Lemaitre, B. Negative Regulation by Amidase PGRPs Shapes the Drosophila Antibacterial Response and Protects the Fly from Innocuous Infection. Immunity 2011, 35, 770–779.
- Morris, O.; Liu, X.; Domingues, C.; Runchel, C.; Chai, A.; Basith, S.; Tenev, T.; Chen, H.; Choi, S.; Pennetta, G.; et al. Signal Integration by the IκB Protein Pickle Shapes Drosophila Innate Host Defense. Cell Host Microbe 2016, 20, 283–295.
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.-M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983.
- Chamy, L.E.; Leclerc, V.; Caldelari, I.; Reichhart, J.-M. Sensing of “danger signals” and pathogen-associated molecular patterns defines binary signaling pathways “upstream” of Toll. Nat. Immunol. 2008, 9, 1165.
- Gottar, M.; Gobert, V.; Matskevich, A.A.; Reichhart, J.-M.; Wang, C.; Butt, T.M.; Belvin, M.; Hoffmann, J.A.; Ferrandon, D. Dual Detection of Fungal Infections in Drosophila via Recognition of Glucans and Sensing of Virulence Factors. Cell 2006, 127, 1425–1437.
- Issa, N.; Guillaumot, N.; Lauret, E.; Matt, N.; Schaeffer-Reiss, C.; Van Dorsselaer, A.; Reichhart, J.-M.; Veillard, F. The Circulating Protease Persephone Is an Immune Sensor for Microbial Proteolytic Activities Upstream of the Drosophila Toll Pathway. Mol. Cell 2018, 69, 539–550.
- Huang, H.-R.; Chen, Z.J.; Kunes, S.; Chang, G.-D.; Maniatis, T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 8322–8327.
- Horng, T.; Medzhitov, R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12654.
- Sun, H.; Bristow, B.N.; Qu, G.; Wasserman, S.A. A heterotrimeric death domain complex in Toll signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 12871.
- Tauszig-Delamasure, S.; Bilak, H.; Capovilla, M.; Hoffmann, J.A.; Imler, J.-L. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat. Immunol. 2001, 3, 91.
- Xiao, T.; Towb, P.; Wasserman, S.A.; Sprang, S.R. Three-Dimensional Structure of a Complex between the Death Domains of Pelle and Tube. Cell 1999, 99, 545–555.
- Moncrieffe, M.C.; Grossmann, J.G.; Gay, N.J. Assembly of Oligomeric Death Domain Complexes during Toll Receptor Signaling. J. Biol. Chem. 2008, 283, 33447–33454.
- Wu, L.P.; Anderson, K.V. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 1998, 392, 93.
- Daigneault, J.; Klemetsaune, L.; Wasserman, S.A. The IRAK homolog Pelle is the functional counterpart of IκB kinase in the Drosophila Toll pathway. PLoS ONE 2013, 8, e75150.
- Roth, S.; Stein, D.; Nüsslein-Volhard, C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 1989, 59, 1189–1202.
- Rushlow, C.A.; Han, K.; Manley, J.L.; Levine, M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 1989, 59, 1165–1177.
- Steward, R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 1989, 59, 1179–1188.
- Imler, J.-L.; Hoffmann, J.A. Toll receptors in innate immunity. Trends Cell Biol. 2001, 11, 304–311.
- De Gregorio, E.; Han, S.-J.; Lee, W.-J.; Baek, M.-J.; Osaki, T.; Kawabata, S.-I.; Lee, B.-L.; Iwanaga, S.; Lemaitre, B.; Brey, P.T. An Immune-Responsive Serpin Regulates the Melanization Cascade in Drosophila. Dev. Cell 2002, 3, 581–592.
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll Signaling Pathway. J. Immunol. 2011, 186, 649.
- Tanji, T.; Yun, E.-Y.; Ip, Y.T. Heterodimers of NF-κB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 14715.
- Ji, S.; Sun, M.; Zheng, X.; Li, L.; Sun, L.; Chen, D.; Sun, Q. Cell-surface localization of Pellino antagonizes Toll-mediated innate immune signalling by controlling MyD88 turnover in Drosophila. Nat. Commun. 2014, 5, 3458.
- Haghayeghi, A.; Sarac, A.; Czerniecki, S.; Grosshans, J.; Schöck, F. Pellino enhances innate immunity in Drosophila. Mech. Dev. 2010, 127, 301–307.
- Goto, A.; Matsushita, K.; Gesellchen, V.; Chamy, L.E.; Kuttenkeuler, D.; Takeuchi, O.; Hoffmann, J.A.; Akira, S.; Boutros, M.; Reichhart, J.M. Akirins are highly conserved nuclear proteins required for NF-κB-dependent gene expression in drosophila and mice. Nat. Immunol. 2007, 9, 97.
- Tartey, S.; Matsushita, K.; Vandenbon, A.; Ori, D.; Imamura, T.; Mino, T.; Standley, D.M.; Hoffmann, J.A.; Reichhart, J.-M.; Akira, S.; et al. Akirin2 is critical for inducing inflammatory genes by bridging IκB-ζ and the SWI/SNF complex. EMBO J. 2014, 33, 2332.
- Nowak, S.J.; Baylies, M.K. Akirin: A context-dependent link between transcription and chromatin remodeling. Bioarchitecture 2012, 2, 209–213.
- Cammarata-Mouchtouris, A.; Nguyen, X.-H.; Acker, A.; Bonnay, F.; Goto, A.; Orian, A.; Fauvarque, M.-O.; Boutros, M.; Reichhart, J.-M.; Matt, N. Hyd ubiquitinates the NF-κB co-factor Akirin to operate an effective immune response in Drosophila. PLOS Pathog. 2020, 16, e1008458.
- Bonnay, F.; Nguyen, X.-H.; Cohen-Berros, E.; Troxler, L.; Batsche, E.; Camonis, J.; Takeuchi, O.; Reichhart, J.-M.; Matt, N. Akirin specifies NF-κB selectivity of Drosophila innate immune response via chromatin remodeling. EMBO J. 2014, 33, 2349–2362.
- Hildebrand, D.G.; Alexander, E.; Hörber, S.; Lehle, S.; Obermayer, K.; Münck, N.-A.; Rothfuss, O.; Frick, J.-S.; Morimatsu, M.; Schmitz, I.; et al. IκBζ Is a Transcriptional Key Regulator of CCL2/MCP-1. J. Immunol. 2013, 190, 4812.
- Kannan, Y.; Yu, J.; Raices, R.M.; Seshadri, S.; Wei, M.; Caligiuri, M.A.; Wewers, M.D. IκBζ augments IL-12– and IL-18–mediated IFN-γ production in human NK cells. Blood 2011, 117, 2855–2863.
- Kohda, A.; Yamazaki, S.; Sumimoto, H. DNA element downstream of the κB site in the Lcn2 promoter is required for transcriptional activation by IκBζ and NF-κB p50. Genes Cells 2014, 19, 620–628.
- Yamamoto, M.; Yamazaki, S.; Uematsu, S.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Kuwata, H.; Takeuchi, O.; Takeshige, K.; et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 2004, 430, 218.
- Naranjo, V.; Ayllón, N.; Pérez de la Lastra, J.M.; Galindo, R.C.; Kocan, K.M.; Blouin, E.F.; Mitra, R.; Alberdi, P.; Villar, M.; de la Fuente, J. Reciprocal Regulation of NF-kB (Relish) and Subolesin in the Tick Vector, Ixodes scapularis. PLoS ONE 2013, 8, e65915.
- Polanowska, J.; Chen, J.-X.; Soulé, J.; Omi, S.; Belougne, J.; Taffoni, C.; Pujol, N.; Selbach, M.; Zugasti, O.; Ewbank, J.J. Evolutionary plasticity in the innate immune function of Akirin. PLoS Genet. 2018, 14, e1007494.
- Bosch, P.J.; Peek, S.L.; Smolikove, S.; Weiner, J.A. Akirin proteins in development and disease: Critical roles and mechanisms of action. Cell. Mol. Life Sci. 2020, 77, 4237–4254.
- Valanne, S.; Järvelä-Stölting, M.; Harjula, S.-K.E.; Myllymäki, H.; Salminen, T.S.; Rämet, M. Osa-Containing Brahma Complex Regulates Innate Immunity and the Expression of Metabolic Genes in Drosophila. J. Immunol. 2020, 204, 2143.
- Nowak, S.J.; Aihara, H.; Gonzalez, K.; Nibu, Y.; Baylies, M.K. Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis. PLoS Genet. 2012, 8, e1002547.
- Fukuyama, H.; Verdier, Y.; Guan, Y.; Makino-Okamura, C.; Shilova, V.; Liu, X.; Maksoud, E.; Matsubayashi, J.; Haddad, I.; Spirohn, K.; et al. Landscape of protein–protein interactions in Drosophila immune deficiency signaling during bacterial challenge. Proc. Natl. Acad. Sci. USA 2013, 110, 10717.
- Goto, A.; Fukuyama, H.; Imler, J.-L.; Hoffmann, J.A. The Chromatin Regulator DMAP1 Modulates Activity of the Nuclear Factor κB Transcription Factor Relish in the Drosophila Innate Immune Response. J. Biol. Chemist. 2014, 289, 20470–20476.
- He, X.; Yu, J.; Wang, M.; Cheng, Y.; Han, Y.; Yang, S.; Shi, G.; Sun, L.; Fang, Y.; Gong, S.; et al. Bap180/Baf180 is required to maintain homeostasis of intestinal innate immune response in Drosophila and mice. Nat. Microbiol. 2017, 2, 17056.
- de Almeida, M.; Hinterndorfer, M.; Brunner, H.; Grishkovskaya, I.; Singh, K.; Schleiffer, A.; Jude, J.; Deswal, S.; Kalis, R.; Vunjak, M.; et al. AKIRIN2 controls the nuclear import of proteasomes in vertebrates. Nature 2021, 599, 491–496.
This entry is offline, you can click here to edit this entry!
