Oxygen (O2) is the most crucial substrate for numerous biochemical processes in plants. Its deprivation is a critical factor that affects plant growth and may lead to death if it lasts for a long time. However, various biotic and abiotic factors cause O2 deprivation, leading to hypoxia and anoxia in plant tissues. To survive under hypoxia and/or anoxia, plants deploy various mechanisms such as fermentation paths, reactive oxygen species (ROS), reactive nitrogen species (RNS), antioxidant enzymes, aerenchyma, and adventitious root formation, while nitrate (NO3−), nitrite (NO2−), and nitric oxide (NO) have shown numerous beneficial roles through modulating these mechanisms. However, the end product of nitrate-nitrite-nitric oxide pathway, the NO is toxic if accumulated. Thus, its scavenging or inhibition is equally important for plant survival. Here, we point out NO reduction to nitrous oxide (N2O) could play an important role in reducing NO toxicity in plants, thus, reducing nitro-oxidative stress and helping plants to survive for longer period during O2-limited conditions
- plants
- hypoxia and anoxia
- nitric oxide signaling
- nitric oxide toxicity
- nitrous oxide
1. Introduction
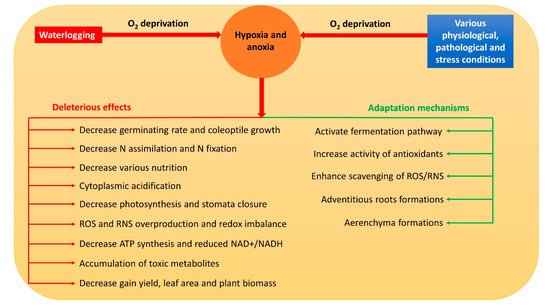
Hypoxia and anoxia result in the modification of various normal metabolic paths [7]. Thus, they usually inhibit respiration, photosynthesis, nitrogen assimilation, biological nitrogen fixation, water and nutrient uptake, and stomata closure in plants [7][8][9][10][11] through a reduced adenosine triphosphate (ATP) concentration, nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide hydrogen (NADH) ratio (NAD+/NADH), and cell viability [12]. Meanwhile, the accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is triggered, which severely damages the cell components [13]. Moreover, during hypoxia and anoxia, a drop in pH causes cytoplasmic acidosis which affects numerous metabolic activities that may even contribute to plant death [14]. Overall, hypoxia and anoxia have numerous deleterious effects on plant metabolism (Figure 1).
2. Pathways of NO Formation during Hypoxia and Anoxia
3. Role of Nitrate and Nitrate Reductase (NR) during Hypoxia and Anoxia Tolerance
Nitrate is not only an important form of nitrogen (N) source to plants but also a signaling molecule [39]. It is usually a major form of N in aerobic soil, and its uptake by plant roots is achieved through NO3− transporters [40]. After being uptaken by roots, NO3− is reduced to NO2− by an enzyme called NR in the cytosol or plasma membrane or stored in the vacuole or transported to shoots and leaves for subsequent reduction [15]. Under normoxia, NO2− is transported to plastids/chloroplasts and is reduced to ammonium (NH4+) by nitrite reductase (NiR, EC 1.7.7.1). Then, glutamine synthetase/glutamate-oxoglutarate aminotransferase (GS, EC 6.3.1.2)/GOGAT, EC 1.4.1.13) assimilates NH4+ into amino acids. However, during hypoxia and anoxia, the NO3− or NH4+ assimilation path to amino acid as well as NO3− transport to the aerial parts is greatly reduced [41]. For example, O2 deficiency decreases NO3− and NH4+ assimilation and N incorporation into amino acids in various plant species as compared to normoxia [42][43]. Although N incorporation into amino acids is inhibited during O2 deficiency, several pieces of research have shown that NR is highly activated and NO3− is reduced to NO2− [44].
Several previous studies have shown that NO3− and NR are beneficial for hypoxia and anoxia tolerance. Germinating seeds generally experience hypoxic and anoxic conditions [45][46][47] due to the compaction and hindrance of O2 diffusion by the outermost layers of seeds [48]. Studies have reported that NO3− is beneficial during seed germination. For example, supplementation or priming with NO3− increases the viability of germination in seeds of various plants [49][50][51]. Light and temperature influence seed germination, while NO3− can reduce the dependency on environmental factors such as light [52] and temperature [53] during germination. Moreover, NO3− can promote germination in seeds during salt, metal, and heat stresses [54][55][56]. The mechanisms of seed germination by NO3− might be due to NO production in cytosol and mitochondria through the reductive pathways [47]. Similarly, NO3− has been shown to increase activities of antioxidant enzymes such as catalase (CAT, EC 1.11.1.6) and superoxide dismutase (SOD, EC 1.15.1.1) during the germination process [57], which could scavenge ROS, thus preventing oxidative damage and promoting germination.
Waterlogging reduces several nutrients in plants, affecting plant metabolism [58], while the supplementation of NO3− increases the uptake of nutrients such as N, P, Fe, and Mn [59]. Nitrate can improve cytoplasmic acidification caused by anoxia in plants [60][61] while decreasing fermentative enzymes such as lactate dehydrogenase (LDH, EC 1.1.1.27), pyruvate decarboxylase (PDC, EC 4.1.1.1), and alcohol dehydrogenase (ADH, EC 1.1.1.1) [62]. Lower levels of lactate and ethanol in plant roots [10][62] and an increase in the ATP level were observed in NO3−-treated plants during waterlogging [62], which suggest that NO3− is highly beneficial to reducing toxic metabolites while increasing the energy status of waterlogged plants. Antioxidants such as SOD, CAT, ascorbate peroxidase (APX, EC 1.11.1.11), and guaiacol peroxidase (POD, EC 1.11.1.7) remove O2− and H2O2 [63][64].
Hypoxia and anoxia in roots caused by flooding decrease chlorophyll content in the leaves of plants, thus decreasing the plant biomass and photosynthesis rate [11]. Nitrate is more beneficial in terms of biomass, net photosynthesis rate, chlorophyll, and protein content as compared to NH4+ and glycine [65][66]. Moreover, the concentration of metabolites such as sucrose, γ-aminobutyrate, succinate, and nucleoside triphosphate are reduced significantly in the absence of NO3− during hypoxia in maize root [60]. Alanine aminotransferase (AlaAT, EC 2.6.1.2), via the reversible conversion of pyruvate and glutamate to alanine and 2-oxoglutarate, is involved in carbon and nitrogen metabolism [67]. The foliar spraying of NO3− during waterlogging increases AlaAT and GOGAT activities along with an increase in amino acid in plants [68], suggesting that NO3− is involved in regulating both glycolysis and the TCA cycle during O2 deficiency. Redox imbalance during hypoxia and anoxia directly affects cellular metabolisms [69]. Various studies have reported that NO3− supplementation to hypoxic and anoxic plant tissues can improve the redox state [15][70][71]. For example, NO3− and NR maintain redox balance during hypoxia in cucumber (Cucumis sativus L.) [12].
Nitrate reduction via NR can delay cell death during hypoxia and delay the anoxic symptoms in plants [72], while its inhibition can significantly disturb the growth [71]. Tobacco (Nicotiana tabacum) mutant plants lacking NR reductase are more sensitive to O2 deprivation as compared to wild types by showing symptoms of rapid wilting, more ethanol and lactate production, and less ATP generation [70], suggesting the role of NO3− is due to its reduction to NO2−. NR plays a role in the maintenance of energy status for nitrogen fixation under O2-limited conditions in Medicago truncatula nodules [73]. The use of NR inhibitors in the root system of nodulated alfalfa (Medicago sativa L.) results in a significant decrease in the ATP/ADP ratio under flooding and salinity stresses [5]. Waterlogging significantly degrades membrane lipids [74], while NO3− and NR activity can delay the anoxia-induced degradation of membrane lipids in plant cells [75]. Higher expression of NR in cucumber (Cucumis sativus) than tomato (Lycopersicon esculentum) was associated with a high tolerance of hypoxia in the roots [76]. During hypoxia and anoxia, NR plays an important role in plant biology by regulating NO production by supplying electrons to NOFNiR and truncated hemoglobin [77]. The regulation of NO is critical, as it is a signaling and also toxic molecule if it is accumulated in a higher amount in a cell [78]. Overall, both NO3−and NR are involved in hypoxia and anoxia tolerance with numerous benefits, which suggests that NO2− is also involved in the mechanisms. However, long-term O2 limitation would affect the NR acclivity, thus, again, questioning plants’ survival during O2-limitation conditions. For example, the NR level increases during O2 limitation conditions, while NR-mRNA remains constant during the early hours of O2 limitation and decreases after 48 h [72], suggesting long-term O2 limitation affects its activity. Moreover, NO, which is produced by NR itself, also decreases the level of NR protein through posttranslational modifications and ubiquitylation by affecting amino acids involved in binding the essential flavin adenine dinucleotide (FAD) and molybdenum cofactors [28][79]. Therefore, O2 limitation and a higher level of NO formation would affect NR activity after long-term hypoxia and anoxia, thus, again, affecting plants’ survival. Moreover, a higher concentration of NO3− is reported to affect plant growth through the increased production of NO, thus increasing lipid peroxidation and the H2O2 level [80].
4. Role of Nitrite during Hypoxia and Anoxia Tolerance
5. Role of Nitric Oxide during Hypoxia and Anoxia Tolerance
The role of NO in plant physiology has been described by numerous researchers. The reductive pathway of NO formation in plants is reported to be beneficial in plants as it promotes seed germination, increases biomass and root formation, increases energy status during O2 limitation, promotes tolerance to various biotic and abiotic stresses, and promotes the induction of different defense-related genes, and many others.
6. Adverse Effects of Nitric Oxide and Role of Nitric Oxide Scavenging on Hypoxia and Anoxia Tolerance
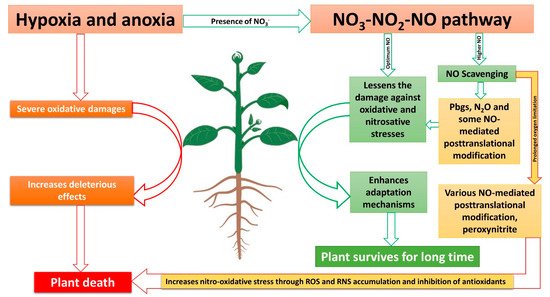
It is clear that NO, as the end product of the NO3-NO2-NO pathway, plays numerous beneficial roles during hypoxia and anoxia tolerance in plants. However, to be beneficial, the concentration of NO plays a critical role, while hypoxia and anoxia trigger NO production, which can be lethal to cells [78]. Some of the adverse effects of NO are summarized in Table 1. Moreover, oxidative stress caused by O2 limitation and the overproduction of NO during various stresses could damage major components of mitochondria [86][116] and inhibit antioxidants systems, thus accumulating ROS and RNS. RNS, if accumulated more, could exacerbate more damage than ROS by triggering free radical peroxidation [117]. Increased RNS and ROS production could lead to retrograde signaling to the nucleus to regulate gene expressions [118]. Nitric oxide, through the formation of RNS, could lead to mutation, DNA damage, and cell death [116][119]. So, for the longer survival of a cell during hypoxia and anoxia, the NO produced RNS should be scavenged efficiently.
| Effects of Higher Level of NO | References |
|---|---|
| Decreases the root growth through DNA damage, induces cell cycle arrest and inhibits primary root growth by affecting root apical meristem activity and cell elongation. | [120][121] |
| Delayed flowering, retarded root development, and reduced starch granule formation through S-nitrosylation modification. | [122] |
| Cell death through increased electrolyte leakage, cell wall degradation, cytoplasmic streaming, and DNA fragmentation. | [21] |
| Decreases the expression of cyclins (CYC) and Cyclin-Dependent Kinases (CDKs), resulting in the downregulation of cell cycle progression. | [123] |
| NO can generate peroxynitrite, which is a mediator of cytochrome c loss, protein oxidation and nitration, lipid peroxidation, mitochondrial dysfunction, damage DNA, and cell death. | [20][124] |
| NO can inhibit antioxidants such as catalase, glutathione peroxidase (GPX), and ascorbate peroxidase in a reversible way and peroxynitrite in an irreversible way. | [96][125] |
| NO can change the redox state and promote cell death. | [26] |
| Inhibits lateral and primary root growth through reduced cell division and the expression of the auxin reporter markers DR5pro:GUS/GFP. | [121][126] |
| Inhibits growth of tobacco plants through peroxynitrite formation and tyrosine nitration. | [127] |
| Inhibits seed germination, while the scavenging of NO alleviates the effect. | [91] |
| Inhibits the shoot growth and decreases the chlorophyll contents of the plants. | [128][129] |
It is clear that NO scavengers work differently in plants. For example, the use of NO scavengers during low NO production have negative effects on plant growth [130], while during high NO production, the same NO scavengers have positive effects [121]. A similar role of NO has been reported in mammals [131]. Therefore, the optimum level of NO could be different during normal and stress conditions. As a higher amount of NO is formed through the reductive pathways during the O2 limitation condition, it would be beneficial that some amount of NO is scavenged from cells. For example, the scavenging of NO using NO scavengers during hypoxia preserves the function of mammals’ mitochondria [132]. There may be several pathways of NO scavenging mechanisms during O2-limited conditions, such as NO reduction to N2O [18][133] and the phytoglobin-NO cycle in plants [134].
NO formation in plants is always suspected to be underestimated [15], which could be due to that fact that NO is not simultaneously measured with N2O. The use of tungsten as an NR inhibitor was reported to inhibit N2O formation in plants [135], while NR inhibition challenged the plants’ survival, as described in the above section, which further supports the concept that N2O formation also could play a role in plants’ survival strategies. Both NO [136] and N2O [137] can increase the activities of phenylalanine ammonialyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA ligase during pathogen attack in plants while increasing total phenolic, flavonoid, and lignin content. Similarly, both NO and N2O are reported to slow down fruit ripening by lowering ethylene synthesis during post-harvest storage [138][139]. Therefore, the similar roles of both NO and N2O observed in plants could be due to NO reduction to N2O, which need further research as, to date, there is no research measuring both NO and N2O simultaneously. This NO reduction could take place in mitochondria and chloroplast of plants cell [18][140][141]. Thus, reducing the toxicity of NO in plants and protecting the components of mitochondria and chloroplast.
As stated in the above sections, the expression of Pgbs is beneficial for plants during O2-limited conditions, which is due to the NO scavenging mechanism. For example, during the germination of barley seeds, the scavenging of NO through the overexpression of Pgbs resulted in a higher germination rate, protein content, and ATP/ADP ratios and a lower rate of fermentation, the S-nitrosylation of proteins and S-nitrosoglutathione (GSNO) [134][142].
7. Nitric-Oxide-Mediated Post-Translational Modifications and Their Roles during Hypoxia and Anoxia
This entry is adapted from the peer-reviewed paper 10.3390/ijms231911522
References
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K.; Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiology and Biochemistry 2020, 148, 228-236, 10.1016/j.plaphy.2020.01.020.
- Greenway, H.; Armstrong, W.; Energy-crises in well-aerated and anoxic tissue: does tolerance require the same specific proteins and energy-efficient transport?. Functional Plant Biology 2018, 45, 877-894, 10.1071/fp17250.
- Valeri, M.C.; Novi, G.; Weits, D.A.; Mensuali, A.; Perata, P.; Loreti, E.; Botrytis cinerea induces local hypoxia in Arabidopsis leaves. New Phytologist 2020, 229, 173-185, 10.1111/nph.16513.
- Serraj, R.; Roy, G.; Drevon, J.J.; Salt stress induces a decrease in the oxygen uptake of soybean nodules and in their permeability to oxygen diffusion. Physiologia Plantarum 1994, 91, 161-168, 10.1111/j.1399-3054.1994.tb00414.x.
- Aridhi, F.; Sghaier, H.; Gaitanaros, A.; Khadri, A.; Aschi-Smiti, S.; Brouquisse, R.; Nitric oxide production is involved in maintaining energy state in Alfalfa (Medicago sativa L.) nodulated roots under both salinity and flooding. Planta 2020, 252, 1-7, 10.1007/s00425-020-03422-1.
- Weits, D.A.; van Dongen, J.T.; Licausi, F.; Molecular oxygen as a signaling component in plant development. New Phytologist 2020, 229, 24-35, 10.1111/nph.16424.
- Limami, A.M.; Diab, H.; Lothier, J.; Nitrogen metabolism in plants under low oxygen stress. Planta 2013, 239, 531-541, 10.1007/s00425-013-2015-9.
- Pugh, R.; Witty, J.F.; Mytton, L.R.; Minchin, F.R.; The effect of waterlogging on nitrogen fixation and nodule morphology in soil-grown white clover (Trifolium repensL.). Journal of Experimental Botany 1995, 46, 285-290, 10.1093/jxb/46.3.285.
- Steffens, D.; Hutsch, B.W.; Eschholz, T.; Losak, T.; Schubert, S.; Water logging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant, Soil and Environment 2011, 51, 545-552, 10.17221/3630-pse.
- Oliveira, H.C.; Freschi, L.; Sodek, L.; Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiology and Biochemistry 2013, 66, 141-149, 10.1016/j.plaphy.2013.02.015.
- Posso, D.A.; Borella, J.; Reissig, G.N.; Bacarin, M.A.; Root flooding-induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiologiae Plantarum 2018, 40, 212, 10.1007/s11738-018-2790-9.
- Shi, K.; Ding, X.-T.; Dong, D.-K.; Zhou, Y.-H.; Yu, J.Q.; Putrescine enhancement of tolerance to root-zone hypoxia in Cucumis sativus: a role for increased nitrate reduction. Functional Plant Biology 2008, 35, 337-345, 10.1071/fp08029.
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J.; Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182, 10.1016/j.plgene.2019.100182.
- Greenway, H.; Gibbs, J.; Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 2003, 30, 999-1036, 10.1071/pp98096.
- Igamberdiev, A.U.; Hill, R.D.; Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. Journal of Experimental Botany 2004, 55, 2473-2482, 10.1093/jxb/erh272.
- Groß, F.; Durner, J.; Gaupels, F.; Nitric oxide, antioxidants and prooxidants in plant defence responses. Frontiers in Plant Science 2013, 4, 419, 10.3389/fpls.2013.00419.
- Gupta, K.J.; Mur, L.A.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G.; The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytologist 2019, 225, 1143-1151, 10.1111/nph.15969.
- Timilsina, A.; Zhang, C.; Pandey, B.; Bizimana, F.; Dong, W.; Hu, C.; Potential Pathway of Nitrous Oxide Formation in Plants. Frontiers in Plant Science 2020, 11, 1177, 10.3389/fpls.2020.01177.
- Corpas, F.J.; Barroso, J.B.; Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytologist 2013, 199, 633-635, 10.1111/nph.12380.
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C.; The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61-72, 10.1016/j.niox.2019.04.005.
- Wany, A.; Kumari, A.; Gupta, K.J.; Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant, Cell & Environment 2017, 40, 3002-3017, 10.1111/pce.13061.
- Lindermayr, C.; Saalbach, G.; Bahnweg, G.; Durner, J.; Differential Inhibition of Arabidopsis Methionine Adenosyltransferases by Protein S-Nitrosylation. Journal of Biological Chemistry 2006, 281, 4285-4291, 10.1074/jbc.m511635200.
- Bai, T.; Li, C.; Ma, F.; Shu, H.; Han, M.; Exogenous Salicylic Acid Alleviates Growth Inhibition and Oxidative Stress Induced by Hypoxia Stress in Malus robusta Rehd. Journal of Plant Growth Regulation 2009, 28, 358-366, 10.1007/s00344-009-9104-9.
- Pande, A.; Mun, B.G.; Rahim, W.; Khan, M.; Lee, D.S.; Lee, G.M.; Al Azzawi, T.N.I.; Hussain, A.; Kim, C.K.; Yun, B.W.; et al. Phytohormonal Regulation Through Protein S-Nitrosylation Under Stress. Frontiers in Plant Science 2022, 13, 865542, 10.3389/fpls.2022.865542.
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J.; Nitric oxide regulation of plant metabolism. Molecular Plant 2021, 15, 228-242, 10.1016/j.molp.2021.12.012.
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T.; On the origins of nitric oxide. Trends in Plant Science 2011, 16, 160-168, 10.1016/j.tplants.2010.11.007.
- Kumari, A.; Pathak, P.K.; Bulle, M.; Igamberdiev, A.U.; Gupta, K.J.; Alternative oxidase is an important player in the regulation of nitric oxide levels under normoxic and hypoxic conditions in plants. Journal of Experimental Botany 2019, 70, 4345-4354, 10.1093/jxb/erz160.
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, Á.; Ocaña-Calahorro, F.; Mariscal, V.; Carreras, A.; Barroso, J.B.; Galván, A.; Fernández, E. A; A dual system formed by the ARC and NR molybdoenzymes mediates nitrite-dependent NO production in Chlamydomonas. Plant, Cell & Environment 2016, 39, 2097-2107, 10.1111/pce.12739.
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Fernández-Ocaña, A.; Chaki, M.; Luque, F.; Barroso, J.B.; Nitrosative stress in plants. FEBS Letters 2007, 581, 453-461, 10.1016/j.febslet.2007.01.006.
- Reda, M.; Golicka, A.; Kabała, K.; Janicka, M.; Involvement of NR and PM-NR in NO biosynthesis in cucumber plants subjected to salt stress. Plant Science 2018, 267, 55-64, 10.1016/j.plantsci.2017.11.004.
- Huang, X.; Stettmaier, K.; Michel, C.; Hutzler, P.; Mueller, M.J.; Durner, J.; Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 2004, 218, 938-946, 10.1007/s00425-003-1178-1.
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang,W.H.; Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiology 2009, 151, 755-767, 10.1104/pp.109.140996.
- Oliveira, H.C.; Saviani, E.E.; Oliveira, J.F.; Salgado, I.; Nitrate reductase-dependent nitric oxide synthesis in the defense response of Arabidopsis thaliana against Pseudomonas syringae. Tropical Plant Pathology 2010, 35, 104-107, 10.1590/s1982-56762010000200005.
- Zhang, M.; Dong, J.-F.; Jin, H.-H.; Sun, L.-N.; Xu, M.-J.; Ultraviolet-B-induced flavonoid accumulation in Betula pendula leaves is dependent upon nitrate reductase-mediated nitric oxide signaling. Tree Physiology 2011, 31, 798-807, 10.1093/treephys/tpr070.
- Monreal, J.A.; Arias-Baldrich, C.; Tossi, V.; Feria, A.B.; Rubio-Casal, A.; García-Mata, C.; García-Mauriño, S.; Nitric oxide regulation of leaf phosphoenolpyruvate carboxylase-kinase activity: implication in sorghum responses to salinity. Planta 2013, 238, 859-869, 10.1007/s00425-013-1933-x.
- Pissolato, M.D.; Silveira, N.M.; Prataviera, P.J.C.; Machado, E.C.; Seabra, A.B.; Pelegrino, M.T.; Sodek, L.; Ribeiro, R.V.; Enhanced Nitric Oxide Synthesis Through Nitrate Supply Improves Drought Tolerance of Sugarcane Plants. Frontiers in Plant Science 2020, 11, 970, 10.3389/fpls.2020.00970.
- Jasid, S.; Simontacchi, M.; Bartoli, C.G.; Puntarulo, S.; Chloroplasts as a Nitric Oxide Cellular Source. Effect of Reactive Nitrogen Species on Chloroplastic Lipids and Proteins. Plant Physiology 2006, 142, 1246-1255, 10.1104/pp.106.086918.
- Singh, I.N.; Sullivan, P.G.; Hall, E.D.; Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. Journal of Neuroscience Research 2007, 85, 2216-2223, 10.1002/jnr.21360.
- Zhao, L.; Liu, F.; Crawford, N.M.; Wang, Y.; Molecular Regulation of Nitrate Responses in Plants. International Journal of Molecular Sciences 2018, 19, 2039, 10.3390/ijms19072039.
- Fan, X.; Naz, M.; Fan, X.; Xuan,W.; Miller, A.J.; Xu, G.; Plant nitrate transporters: from gene function to application. Journal of Experimental Botany 2017, 68, 2463-2475, 10.1093/jxb/erx011.
- Brandão, A.D.; Sodek, L.; Nitrate uptake and metabolism by roots of soybean plants under oxygen deficiency.. Brazilian Journal of Plant Physiology 2009, 21, 13-23, 10.1590/s1677-04202009000100003.
- Oliveira, H.C.; Sodek, L.; Effect of oxygen deficiency on nitrogen assimilation and amino acid metabolism of soybean root segments. Amino Acids 2012, 44, 743-755, 10.1007/s00726-012-1399-3.
- Liu, B.; Rennenberg, H.; Kreuzwieser, J.; Hypoxia Affects Nitrogen Uptake and Distribution in Young Poplar (Populus × canescens) Trees. PLOS ONE 2015, 10, e0136579, 10.1371/journal.pone.0136579.
- Botrel, A.; Magné, C.; Kaiser, W.M.; Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol. Biochem 1996, 34, 645–652, .
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D.; Nitrite–nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochimica et Biophysica Acta 2008, 1777, 1268-1275, 10.1016/j.bbabio.2008.06.002.
- Borisjuk, L.; Rolletschek, H.; The oxygen status of the developing seed. New Phytologist 2009, 182, 17-30, 10.1111/j.1469-8137.2008.02752.x.
- Pandey, S.; Kumari, A.; Shree, M.; Kumar, V.; Singh, P.; Bharadwaj, C.; Loake, G.J.; Parida, S.K.; Masakapalli, S.K.; Gupta, K.J.; et al. Nitric oxide accelerates germination via the regulation of respiration in chickpea.. Journal of Experimental Botany 2019, 70, 4539-4555, 10.1093/jxb/erz185.
- Borisjuk, L.; Macherel, D.; Benamar, A.; Wobus, U.; Rolletschek, H.; Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytologist 2007, 176, 813-823, 10.1111/j.1469-8137.2007.02226.x.
- Hendricks, S.B.; Taylorson, R.B.; Promotion of Seed Germination by Nitrates and Cyanides. Nature Cell Biology 1972, 237, 169-170, 10.1038/237169b0.
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevi, M.; Alvarenga, A.A.; Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. Journal of Agricultural Science 2014, 6, p72, 10.5539/jas.v6n2p72.
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. The Plant Cell 2020, 32, 2094-2119, 10.1105/tpc.19.00748.
- Deng, Z.; Song, S.; Sodium nitroprusside, ferricyanide, nitrite and nitrate decrease the thermo-dormancy of lettuce seed germination in a nitric oxide-dependent manner in light. South African Journal of Botany 2012, 78, 139-146, 10.1016/j.sajb.2011.06.009.
- Dong, T.; Tong, J.; Xiao, L.; Cheng, H.; Song, S.; Nitrate, abscisic acid and gibberellin interactions on the thermoinhibition of lettuce seed germination. Plant Growth Regulation 2011, 66, 191-202, 10.1007/s10725-011-9643-5.
- Nawaz, F.; Naeem, M.; Akram, A.; Ashraf, M.Y.; Ahmad, K.S.; Zulfiqar, B.; Sardar, H.; Shabbir, R.N.; Majeed, S.; Shehzad, M.A.; et al. Seed priming with KNO3 mediates biochemical processes to inhibit lead toxicity in maize (Zea mays L.). Journal of the Science of Food and Agriculture 2017, 97, 4780-4789, 10.1002/jsfa.8347.
- Ikeya, S.; Aoyanagi, T.; Ishizuka, I.; Takeuchi, A.; Kozaki, A.; Nitrate Promotes Germination Under Inhibition by NaCl or High Concentration of Glucose. Plants 2020, 9, 707, 10.3390/plants9060707.
- Kumar, V.; Dwivedi, P.; Kumar, P.; Singh, B.N.; Pandey, D.K.; Kumar, V.; Bose, B.; Mitigation of heat stress responses in crops using nitrate primed seeds. South African Journal of Botany 2021, 140, 25-36, 10.1016/j.sajb.2021.03.024.
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevi, M.; Alvarenga, A.A.; Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. Journal of Agricultural Science 2014, 6, 72, 10.5539/jas.v6n2p72.
- Board, J.E.; Waterlogging Effects on Plant Nutrient Concentrations in Soybean. Journal of Plant Nutrition 2008, 31, 828-838, 10.1080/01904160802043122.
- Ashraf, M.; Rehman, H.; Mineral nutrient status of corn in relation to nitrate and long‐term waterlogging. Journal of Plant Nutrition 1999, 22, 1253-1268, 10.1080/01904169909365710.
- Fan, T.W.; Higashi, R.M.; Lane, A.N.; An in vivo1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics 1988, 266, 592-606, 10.1016/0003-9861(88)90292-5.
- Libourel, I.G.L.; Van Bodegom, P.M.; Fricker, M.D.; Ratcliffe, R.G.; Nitrite Reduces Cytoplasmic Acidosis under Anoxia. Plant Physiology 2006, 142, 1710-1717, 10.1104/pp.106.088898.
- Da-Silva, C.J.; do Amarante, L.; Short-term nitrate supply decreases fermentation and oxidative stress caused by waterlogging in soybean plants. Environmental and Experimental Botany 2020, 176, 104078, 10.1016/j.envexpbot.2020.104078.
- Gill, S.S.; Tuteja, N.; Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 2010, 48, 909-930, 10.1016/j.plaphy.2010.08.016.
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V.; Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681, 10.3390/antiox9080681.
- Posso, D.A.; Borella, J.; Reissig, G.N.; do Amarante, L.; Bacarin, M.A.; Nitrate-mediated maintenance of photosynthetic process by modulating hypoxic metabolism of common bean plants. Acta Physiologiae Plantarum 2020, 42, 1-17, 10.1007/s11738-020-03107-y.
- Naz, S.; Shen, Q.; Lwalaba, J.L.W.; Zhang, G.; Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley. Plants 2021, 10, 595, 10.3390/plants10030595.
- Nakamura, M.; Noguchi, K.; Tolerant mechanisms to O2 deficiency under submergence conditions in plants. Journal of Plant Research 2020, 133, 343-371, 10.1007/s10265-020-01176-1.
- Kaur, K.; Goyal, K.; Arora, K.; Kaur, G.; Genotypic variations in nitrate respiration along with potassium nitrate treatment - accountable for water logging tolerance in maize. Biologia 2021, 76, 1651-1660, 10.1007/s11756-021-00749-2.
- Moore, M.; Gossmann, N.; Dietz, K.J.; Redox Regulation of Cytosolic Translation in Plants. Trends in Plant Science 2016, 21, 388-397, 10.1016/j.tplants.2015.11.004.
- Stoimenova, M.; Libourel, I.G.L.; Ratcliffe, R.G.; Kaiser,W.M.; The role of nitrate reduction in the anoxic metabolism of roots II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant and Soil 2003, 253, 155-167, 10.1023/a:1024591116697.
- Horchani, F.; Aschi-Smiti, S.; Brouquisse, R.; Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiologiae Plantarum 2010, 32, 1113-1123, 10.1007/s11738-010-0503-0.
- Allègre, A.; Silvestre, J.; Morard, P.; Kallerhoff, J.; Pinelli, E.; Nitrate reductase regulation in tomato roots by exogenous nitrate: a possible role in tolerance to long-term root anoxia. Journal of Experimental Botany 2004, 55, 2625-2634, 10.1093/jxb/erh258.
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both Plant and Bacterial Nitrate Reductases Contribute to Nitric Oxide Production in Medicago truncatula Nitrogen-Fixing Nodules. Plant Physiology 2010, 155, 1023-1036, 10.1104/pp.110.166140.
- Xu, L.; Pan, R.; Zhang,W.; Membrane lipids are involved in plant response to oxygen deprivation. Plant Signaling & Behavior 2020, 15, 1771938, 10.1080/15592324.2020.1771938.
- Oberson, J.; Pavelic, D.; Braendle, R.; Rawyler, A.; Nitrate Increases Membrane Stability of Potato Cells under Anoxia. Journal of Plant Physiology 1999, 155, 792-794, 10.1016/s0176-1617(99)80098-4.
- Guo, S.R.; Nada, K.; Tachibana, S. A; A Role for Nitrate Reductase in the High Tolerance of Cucumber Seedlings to Root-zone Hypoxia.. Journal of the Japanese Society for Horticultural Science 1998, 67, 613-618, 10.2503/jjshs.67.613.
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E.; Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends in Plant Science 2017, 22, 163-174, 10.1016/j.tplants.2016.12.001.
- Brown, G.C.; Borutaite, V.; Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radical Biology and Medicine 2002, 33, 1440-1450, 10.1016/s0891-5849(02)01112-7.
- Costa-Broseta, Á.; Castillo, M.; León, J.; Post-Translational Modifications of Nitrate Reductases Autoregulates Nitric Oxide Biosynthesis in Arabidopsis. International Journal of Molecular Sciences 2021, 22, 549, 10.3390/ijms22020549.
- Zheng, P.; Bai, X.; Long, J.; Li, K.; Xu, H.; Nitric oxide enhances the nitrate stress tolerance of spinach by scavenging ROS and RNS. Scientia Horticulturae 2016, 213, 24-33, 10.1016/j.scienta.2016.10.008.
- Gupta, K.J.; Igamberdiev, A.U.; The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 2011, 11, 537-543, 10.1016/j.mito.2011.03.005.
- Bethke, P.C.; Libourel, I.G.; Reinöhl, V.; Jones, R.L.; Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 2005, 223, 805-812, 10.1007/s00425-005-0116-9.
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D.; Nitrite-nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochimica et Biophysica Acta 2008, 1777, 1268-1275, 10.1016/j.bbabio.2008.06.002.
- Oliveira, H.C.; Salgado, I.; Sodek, L.; Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 2013, 237, 255-264, 10.1007/s00425-012-1773-0.
- Gupta, K.J.; Lee, C.P.; Ratcliffe, R.G.; Nitrite Protects Mitochondrial Structure and Function under Hypoxia. Plant and Cell Physiology 2016, 58, pcw174-183, 10.1093/pcp/pcw174.
- Srinivasan, S.; Avadhani, N.G.; Cytochrome c oxidase dysfunction in oxidative stress. Free Radical Biology and Medicine 2012, 53, 1252-1263, 10.1016/j.freeradbiomed.2012.07.021.
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T.; The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery 2008, 7, 156-167, 10.1038/nrd2466.
- Fago, A.; Jensen, F.B.; Hypoxia Tolerance, Nitric Oxide, and Nitrite: Lessons From Extreme Animals. Physiology 2015, 30, 116-126, 10.1152/physiol.00051.2014.
- Ansari, F.A.; Ali, S.N.; Arif, H.; Khan, A.A.; Mahmood, R.; Acute oral dose of sodium nitrite induces redox imbalance, DNA damage, metabolic and histological changes in rat intestine. PLOS ONE 2017, 12, e0175196, 10.1371/journal.pone.0175196.
- Kopyra, M.; Gwó´zd´z, E.; Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiology and Biochemistry 2003, 41, 1011-1017, 10.1016/j.plaphy.2003.09.003.
- Bethke, P.C.; Libourel, I.G.; Jones, R.L.; Nitric oxide reduces seed dormancy in Arabidopsis. Journal of Experimental Botany 2005, 57, 517-526, 10.1093/jxb/erj060.
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J.; Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Annals of Botany 2008, 103, 197-209, 10.1093/aob/mcn211.
- Wu, M.; Wang, F.; Zhang, C.; Xie, Y.; Han, B.; Huang, J.; Shen,W.; Heme oxygenase-1 is involved in nitric oxide- and cGMP-induced α-Amy2/54 gene expression in GA-treated wheat aleurone layers. Plant Molecular Biology 2012, 81, 27-40, 10.1007/s11103-012-9979-x.
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O.; S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nature Communications 2015, 6, 8669, 10.1038/ncomms9669.
- Wang, Z.; Ma, R.; Zhao, M.; Wang, F.; Zhang, N.; Si, H.; NO and ABA Interaction Regulates Tuber Dormancy and Sprouting in Potato. Frontiers in Plant Science 2020, 11, 311, 10.3389/fpls.2020.00311.
- Clark, D.; Durner, J.; Navarre, D.A.; Klessig, D.F.; Nitric Oxide Inhibition of Tobacco Catalase and Ascorbate Peroxidase. Molecular Plant-Microbe Interactions 2000, 13, 1380-1384, 10.1094/mpmi.2000.13.12.1380.
- Peng, R.; Bian, Z.; Zhou, L.; Cheng, W.; Hai, N.; Yang, C.; Yang, T.; Wang, X.; Wang, C.; Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Reports 2016, 35, 2325-2340, 10.1007/s00299-016-2037-4.
- Zhang, Y.; Zhang, Y.; Liu, G.; Xu, S.; Dai, J.; Li,W.; Li, Z.; Zhang, D.; Li, C.; Dong, H.; et al. Nitric oxide increases the biomass and lint yield of field-grown cotton under temporary waterlogging through physiological and molecular regulation. Field Crops Research 2020, 261, 107989, 10.1016/j.fcr.2020.107989.
- Liu, F.; Guo, F.-Q.; Nitric Oxide Deficiency Accelerates Chlorophyll Breakdown and Stability Loss of Thylakoid Membranes during Dark-Induced Leaf Senescence in Arabidopsis. PLOS ONE 2013, 8, e56345, 10.1371/journal.pone.0056345.
- Wang, B.; Liu, H.; Li, C.; Zhu, Y.; Tian, X.; Ma, G.; Zou, H.; Effects of nitric oxide on some physiological characteristics of maize seedlings under waterlogging. African Journal of Agricultural Research 2011, 6 (19), 4501-4504, 10.5897/AJAR11.1029.
- Pan, Q.-N.; Geng, C.-C.; Li, D.-D.; Xu, S.-W.; Mao, D.-D.; Umbreen, S.; Loake, G.J.; Cui, B.-M.; Nitrate Reductase-Mediated Nitric Oxide Regulates the Leaf Shape in Arabidopsis by Mediating the Homeostasis of Reactive Oxygen Species. International Journal of Molecular Sciences 2019, 20, 2235, 10.3390/ijms20092235.
- Manoli, A.; Begheldo, M.; Genre, A.; Lanfranco, L.; Trevisan, S.; Quaggiotti, S.; NO homeostasis is a key regulator of early nitrate perception and root elongation in maize*. Journal of Experimental Botany 2013, 65, 185-200, 10.1093/jxb/ert358.
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D.; Nitrite–nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochimica et Biophysica Acta 2008, 1777, 1268-1275, 10.1016/j.bbabio.2008.06.002.
- Gupta, K.J.; Kumari, A.; Florez-Sarasa, I.; Fernie, A.R.; Igamberdiev, A.U.; Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. Journal of Experimental Botany 2018, 69, 3413-3424, 10.1093/jxb/ery119.
- Jayawardhane, J.; Cochrane, D.W.; Vyas, P.; Bykova, N.V.; Vanlerberghe, G.C.; Igamberdiev, A.U.; Roles for Plant Mitochondrial Alternative Oxidase Under Normoxia, Hypoxia, and Reoxygenation Conditions. Frontiers in Plant Science 2020, 11, 566, 10.3389/fpls.2020.00566.
- Rutten, P.J.; Poole, P.S.; Oxygen regulatory mechanisms of nitrogen fixation in rhizobia. Adv. Microb. Physiol. 2019, 75, 325-389, 10.1016/bs.ampbs.2019.08.001.
- Berger, A.; Guinand, S.; Boscari, A.; Puppo, A.; Brouquisse, R.; Medicago truncatula Phytoglobin 1.1 controls symbiotic nodulation and nitrogen fixation via the regulation of nitric oxide concentration. New Phytologist 2020, 227, 84-98, 10.1111/nph.16462.
- He, L.; Li, B.; Lu, X.; Yuan, L.; Yang, Y.; Yuan, Y.; Du, J.; Guo, S.; The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Scientific Reports 2015, 5, 11391, 10.1038/srep11391.
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S.; The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biology 2018, 18, 1-10, 10.1186/s12870-018-1393-3.
- Besson-Bard, A.; Pugin, A.; Wendehenne, D.; New Insights into Nitric Oxide Signaling in Plants. Annual Review of Plant Biology 2008, 59, 21-39, 10.1146/annurev.arplant.59.032607.092830.
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D.; Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radical Biology and Medicine 2006, 40, 1369-1376, 10.1016/j.freeradbiomed.2005.12.006.
- Loitto, V.M.; Nilsson, H.; Sundqvist, T.; Magnusson, K.E.; Nitric oxide induces dose-dependent CA2+ transients and causes temporal morphological hyperpolarization in human neutrophils. Journal of Cellular Physiology 2000, 182, 402-413, 10.1002/(sici)1097-4652(200003)182:3<402::aid-jcp11>3.0.co;2-d.
- Chen, T.; Yuan, F.; Song, J.; Wang, B.; Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Functional Plant Biology 2016, 43, 244, 10.1071/fp15120.
- Rich, S.M.; Ludwig, M.; Pedersen, O.; Colmer, T.D.; Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: implications for root function during flooding. New Phytologist 2010, 190, 311-319, 10.1111/j.1469-8137.2010.03524.x.
- Yamauchi, T.; Tanaka, A.; Tsutsumi, N.; Inukai, Y.; Nakazono, M.; A Role for Auxin in Ethylene-Dependent Inducible Aerenchyma Formation in Rice Roots. Plants 2020, 9, 610, 10.3390/plants9050610.
- Murphy, M.P.; Nitric oxide and cell death. Biochimica et Biophysica Acta 1999, 1411, 401-414, 10.1016/s0005-2728(99)00029-8.
- Deng, Y.; Jia, F.; Chen, S.; Shen, Z.; Jin, Q.; Fu, G.; Ji, J.; Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials 2018, 187, 55-65, 10.1016/j.biomaterials.2018.09.043.
- Hebelstrup, K.H.; Møller, I.M.; Mitochondrial Signaling in Plants Under Hypoxia: Use of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants 2014, 1, 63-77, 10.1007/978-3-319-10079-1_4.
- Nguyen, T.; Brunson, D.; Crespi, C.L.; Penman, B.W.; Wishnok, J.S.; Tannenbaum, S.R.; DNA damage and mutation in human cells exposed to nitric oxide in vitro.. Proceedings of the National Academy of Sciences 1992, 89, 3030-3034, 10.1073/pnas.89.7.3030.
- Bai, S.; Li, M.; Yao, T.; Wang, H.; Zhang, Y.; Xiao, L.; He, Y.; Nitric oxide restrain root growth by DNA damage induced cell cycle arrest in Arabidopsis thaliana. Nitric Oxide 2011, 26, 54-60, 10.1016/j.niox.2011.12.001.
- Fernández-Marcos, M.; Sanz, L.; Lewis, D.R.; Muday, G.K.; Lorenzo, O.; Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proceedings of the National Academy of Sciences 2011, 108, 18506-18511, 10.1073/pnas.1108644108.
- Zhang, Z.-W.; Luo, S.; Zhang, G.-C.; Feng, L.-Y.; Zheng, C.; Zhou, Y.-H.; He, Y.-K.; Nitric oxide induces monosaccharide accumulation through enzyme S-nitrosylation. Plant, Cell & Environment 2017, 40, 1834-1848, 10.1111/pce.12989.
- Novikova, G.V.; Mur, L.A.; Nosov, A.V.; Fomenkov, A.A.; Mironov, K.S.; Mamaeva, A.S.; Hall, M.A.; Nitric Oxide Has a Concentration-Dependent Effect on the Cell Cycle Acting via EIN2 in Arabidopsis thaliana Cultured Cells. Frontiers in Physiology 2017, 8, 142, 10.3389/fphys.2017.00142.
- Vandelle, E.; Delledonne, M.; Peroxynitrite formation and function in plants. Plant Science 2011, 181, 534-539, 10.1016/j.plantsci.2011.05.002.
- Castella, C.; Mirtziou, I.; Seassau, A.; Boscari, A.; Montrichard, F.; Papadopoulou, K.; Brouquisse, R.; Post-translational modifications of Medicago truncatula glutathione peroxidase 1 induced by nitric oxide. Nitric Oxide 2017, 68, 125-136, 10.1016/j.niox.2017.02.004.
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L.; Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900-905, 10.1007/s00425-003-1172-7.
- Morot-Gaudry-Talarmain, Y.; Rockel, P.; Moureaux, T.; Quillere, I.; Leydecker, M.; Kaiser, W.; Morot-Gaudry, J.; Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta 2002, 215, 708-715, 10.1007/s00425-002-0816-3.
- Lazalt, A.M.; Beligni, M.V.; Lamattina, L.; Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. European Journal of Plant Pathology 1997, 103, 643-651, 10.1023/a:1008604410875.
- He, Y.; Tang, R.-H.; Hao, Y.; Stevens, R.-D.; Cook, C.-W.; Ahn, S.-M.; Pei, Z.-M.; Nitric Oxide Represses the Arabidopsis Floral Transition. Science 2004, 305, 1968-1971, 10.1126/science.1098837.
- Sun, H.; Feng, F.; Liu, J.; Zhao, Q.; Nitric Oxide Affects Rice Root Growth by Regulating Auxin Transport Under Nitrate Supply. Frontiers in Plant Science 2018, 9, 659, 10.3389/fpls.2018.00659.
- Phoa, N.; Epe, B.; Influence of nitric oxide on the generation and repair of oxidative DNA damage in mammalian cells.. Carcinogenesis 2002, 23, 469-475, 10.1093/carcin/23.3.469.
- Emmanuel, R.; Alexandre, D.; Benoit, V.; Mohamed, H.S.; Remi, N.; Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation. Pharmacological Reports 2011, 63, 1189-1194, 10.1016/s1734-1140(11)70638-7.
- Timilsina, A.; Bizimana, F.; Pandey, B.; Yadav, R.K.P.; Dong, W.; Hu, C.; Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants. Plants 2020, 9, 180, 10.3390/plants9020180.
- Cochrane, D.W.; Shah, J.K.; Hebelstrup, K.H.; Igamberdiev, A.U.; Expression of phytoglobin affects nitric oxide metabolism and energy state of barley plants exposed to anoxia. Plant Science 2017, 265, 124-130, 10.1016/j.plantsci.2017.10.001.
- Goshima, N.; Mukai, T.; Suemori, M.; Takahashi, M.; Caboche, M.; Morikawa, H.; Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense NiR mRNA. The Plant Journal 1999, 19, 75-80, 10.1046/j.1365-313x.1999.00494.x.
- Li, G.; Zhu, S.; Wu, W.; Zhang, C.; Peng, Y.; Wang, Q.; Shi, J.; Exogenous nitric oxide induces disease resistance againstMonilinia fructicolathrough activating the phenylpropanoid pathway in peach fruit. Journal of the Science of Food and Agriculture 2016, 97, 3030-3038, 10.1002/jsfa.8146.
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B.; Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Scientia Horticulturae 2019, 254, 99-105, 10.1016/j.scienta.2019.04.061.
- Palomer, X.; Roig-Villanova, I.; Grima-Calvo, D.; Vendrell, M.; Effects of nitrous oxide (N2O) treatment on the postharvest ripening of banana fruit. Postharvest Biology and Technology 2005, 36, 167-175, 10.1016/j.postharvbio.2004.12.008.
- Zhu, S.-H.; Zhou, J.; Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chemistry 2007, 100, 1517-1522, 10.1016/j.foodchem.2005.12.022.
- Timilsina, A.; Oenema, O.; Luo, J.; Wang, Y.; Dong, W.; Pandey, B.; Hu, C.; Plants are a natural source of nitrous oxide even in field conditions as explained by 15N site preference. Science of The Total Environment 2021, 805, 150262, 10.1016/j.scitotenv.2021.150262.
- Smart, D.R.; Bloom, A.J.; Wheat leaves emit nitrous oxide during nitrate assimilation. Proceedings of the National Academy of Sciences 2001, 98, 7875-7878, 10.1073/pnas.131572798.
- Zafari, S.; Hebelstrup, K.H.; Igamberdiev, A.U.; Transcriptional and Metabolic Changes Associated with Phytoglobin Expression during Germination of Barley Seeds. International Journal of Molecular Sciences 2020, 21, 2796, 10.3390/ijms21082796.
- Chaki, M.; Begara-Morales, J.C.; Valderrama, R.; Mata-Pérez, C.; Padilla-Serrano, M.N.; Barroso, J.B.; Role of nitric oxide-dependent posttranslational modifications of proteins under abiotic stress. Plant Life under Changing Environment 2020, 1, 793-809, 10.1016/b978-0-12-818204-8.00035-7.
- Machacova, K.; Papen, H.; Kreuzwieser, J.; Rennenberg, H.; Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant and Soil 2013, 364, 287-301, 10.1007/s11104-012-1359-4.
- Zhao, X.-J.; Sampath, V.; Caughey, W.S.; Cytochrome c Oxidase Catalysis of the Reduction of Nitric Oxide to Nitrous Oxide. Biochemical and Biophysical Research Communications 1995, 212, 1054-1060, 10.1006/bbrc.1995.2076.
- Oliveira, H.C.; Salgado, I.; Role of Plant Mitochondria in Nitric Oxide Homeostasis During Oxygen Deficiency. Nitric Oxide in Plants: Metabolism and Role in Stress Physiology 2014, 1, 57-74, 10.1007/978-3-319-06710-0_4.
- Sturms, R.; DiSpirito, A.A.; Hargrove, M.S.; Plant and Cyanobacterial Hemoglobins Reduce Nitrite to Nitric Oxide under Anoxic Conditions. Biochemistry 2011, 50, 3873-3878, 10.1021/bi2004312.