Malignant transformation due to infectious disease can occur via two broad mechanisms. The agent can act as a direct carcinogen altering the expression of oncogenes; thereby, leading to the production of oncoproteins. These oncoproteins can then interact with cellular proteins, and ultimately lead to mutagenesis by the disruption of cell cycle check-points, inhibition of apoptosis, and enhancement of cell immortalisation. In feline medicine, there have been several viruses which demonstrate direct mutagenesis, these include feline leukaemia virus (FeLV) and mouse mammary tumour virus (MMTV). Alternatively, neoplastic transformation can be driven via indirect mechanisms; these include induction of chronic inflammation, which, in turn, results in the production of inflammatory mediators, and the production of reactive oxygen species, which have direct mutagenic effects and promote tumour neovascularisation. Inflammation-induced neoplasms have been associated with Helicobacter organisms and, potentially, Opisthorchis infections. Furthermore, immune suppression induced by viruses such as feline immunodeficiency virus (FIV), not only predispose to infection with other agents, it is also thought to alter the immune surveillance, and with this, the ability to remove neoplastic cells by the host.
- retrovirus
- papillomavirus
- FeLV
- FIV
- Helicobacter
- lymphoma
- parasitic
1. Viral Infections Associated with Neoplasia in the Cat
1.1. Feline Leukaemia Virus (FeLV)
Since its discovery in 1964, FeLV has been reported globally [1]. It is recognised that progressive infection with FeLV has been shown to increase the risk of lymphoma development by 60 times and is associated with the development of other forms of neoplasia [2][3]. At its peak, it was estimated that approximately one-third of all cancer related deaths in cats were attributable to FeLV infection [4]. However, today the prevalence of FeLV infection is markedly decreased [5].
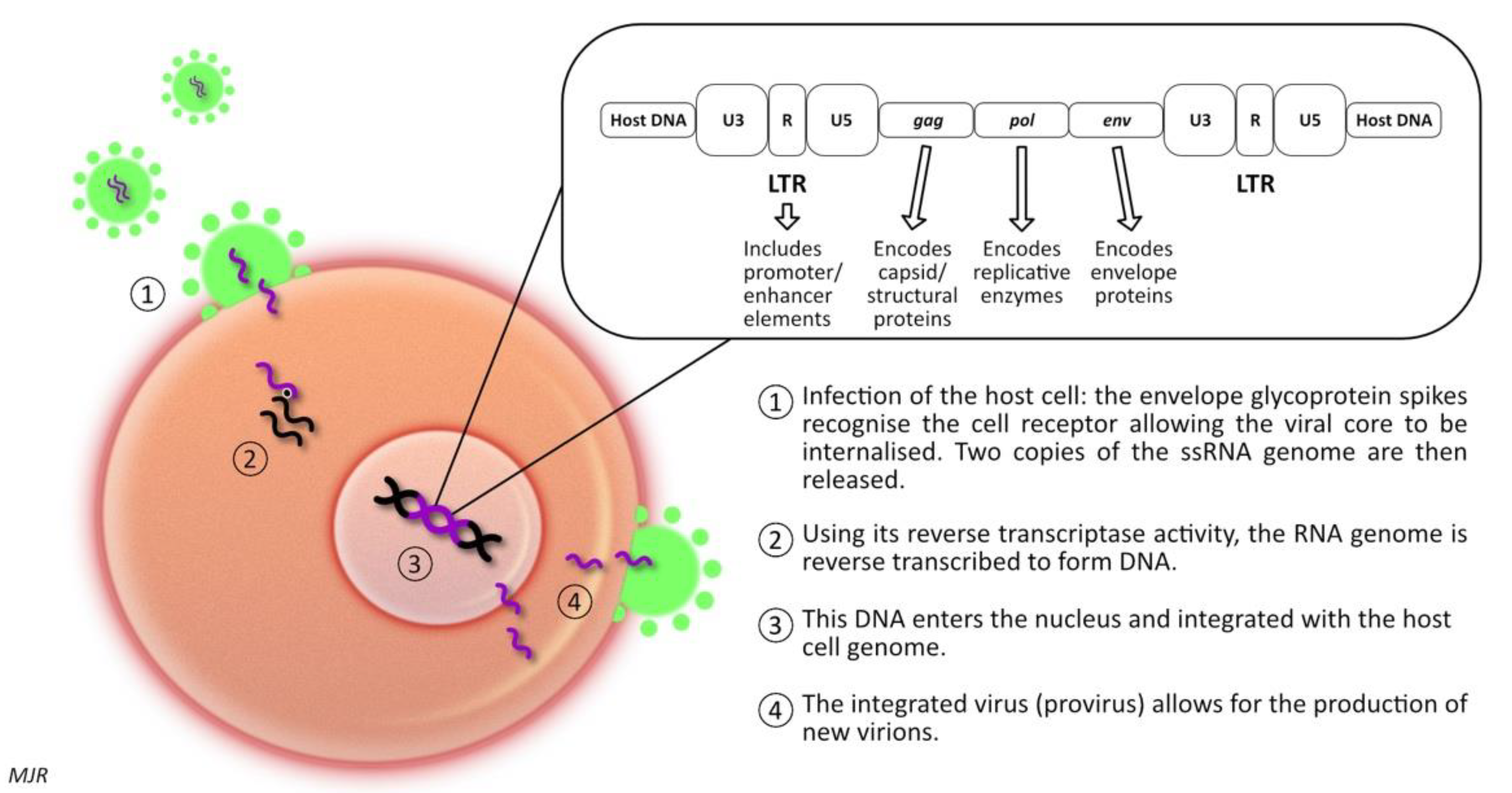
1.2. Feline Immunodeficiency Virus (FIV)
1.3. FIV & Gammaherpesviruses
1.4. Mouse Mammary Tumour Virus (MMTV)
MMTV is an oncogenic retrovirus that induces mammary carcinoma in mice and for which, possible links to human breast cancer have been suggested [92]. MMTV is a beta-retrovirus, composed of prominent surface spikes with an eccentric condensed core. The genome codes for both structural and non-structural proteins, and therefore, MMTV is classified as a complex retrovirus [92][93]. The transcript codes for Gag, Pol, pro-dUTPase (DUT) protein and Env, flanked by LTRs [92]. These LTRs are exceptionally long, and encode for two additional genes: sag (a viral accessory protein that functions as a superantigen) and rem (which encodes an RNA export protein) [92]. MMTV replicates efficiently in the mammary alveolar epithelial cells, with increased expression observed during lactation due to the release of steroid hormones [92]. In mice, lactogenic transmission results in the virus infecting the dendritic cells and B lymphocytes of the Peyer’s patches. The sag antigen is then presented to CD4+ T-lymphocytes. This protein triggers viral replication and amplification of T-lymphocytes, which act as a carrier to transfer the virus from the gut to the mammary tissue. MMTV infection does not immediately trigger neoplastic transformation, even once proviral DNA is inserted in the cell DNA [92]. The insertion results in proto-oncogene deregulation (much like infection with FeLV). To date, rearrangements in several cellular gene families (wnt, fgf, notch4/int3, rspo and the gene encoding the p48 component of eukaryotic translation initiation factor-3 (elF-3p48)) have been demonstrated with MMTV infection [92][94]. It is hypothesised that these insertions are responsible for neoplastic transformation. However, indirect mechanisms for MMTV tumorigenesis have also been implicated [95]. It has been suggested that MMTV may activate an immunoreceptor tyrosine-based mechanism that suppresses apoptosis [95]. Alternatively, it has been hypothesised that MMTV infection could lead to the activation of other infections, such as EBV or HPV, which have also been implicated in the development of mammary neoplasia [95].
1.5. Papillomaviruses
One of the strongest associations between an infectious agent and neoplasia in humans is the role of papillomaviruses in cervical carcinoma [96]. There are currently six different feline papillomaviruses recognised; Felis catus papillomavirus types 1-6 (FcaPV 1-6) [97]. Cats are also dead-end hosts for the delta-papillomavirus Bos taurus papillomavirus-14 (BPV-14) [97]. The majority of cats are infected with FcaPV’s, but disease associated with infection is rare, suggesting that, in most circumstances, host defences inhibit viral replication [98]. Occasionally, papillomaviruses can induce a rapid (self-limited) increase in cell growth, resulting in a hyperplastic papilloma (wart) or a more modest increase in cell growth, leading to the development of a raised plaque [99]. The increased replication within cells can lead to neoplastic transformation [99]. There are various cutaneous lesions that have been associated with papillomavirus infection in cats. These include, cutaneous papilloma’s (warts), which are rare in the cat; viral plaques and Bowenoid in situ carcinoma (BISC); feline cutaneous squamous cell carcinoma (SCC); basal cell carcinoma (BCC), feline sarcoid, and Merkle cell carcinoma [100][101][102]. In addition, oral papilloma’s and oral squamous cell carcinoma (OSCC) have been linked to feline papillomavirus infection [97].
Feline viral plaques and BISC are thought to represent two extremes of the same disease process [98]. These lesions typically develop in middle to older aged cats [103]. They can be found anywhere on the body, but are most common around the head and neck. Viral plaques are small (less than 8mm) slightly raised, hairless lesions, whereas BISCs are larger, more markedly raised, ulcerated, and crusted lesions [97]. The lesions are considered pre-cancerous, but can progress to invasive squamous cell carcinoma in some cases [97]. Studies have demonstrated that both viral plaques and BISCs are frequently associated with FcaPV-2 infection [104][105][106][107][108][109][110][111]. The most common cause of viral plaques/BISC is FcaPV-2, which was identified in 48% of BISCs using immunohistochemistry [112].
Cutaneous SCC in cats are most notably associated with UV-light exposure. However, there is evidence that FcaPVs may also play a role. Initially, it was noted that FcaPV-2 was identified more frequently in SCC than in non-SCC skin [113][114]. However, FcaPV-2 E6 and E7 RNA has also been identified in a proportion of SCCs, but not normal skin; more recently, the E6 and E7 proteins have been shown to influence neoplastic transformation [115][116][117]. FcaPVs have been detected in 76% of SCCs occurring in UV-protected (i.e., haired) areas, and 42% of SCCs from UV-exposed skin [118]. Furthermore, p16 has been identified in 84% of UV-protected SCC and 40% of UV-exposed SCC [104][118].
1.6. Hepadnavirus
2. Bacterial Causes of Neoplasia
In cats, several enterohepatic Helicobacter species have been recognised. A study in 2001 demonstrated Helicobacter species in 17 of 45 cats [137]. Of these, nine were demonstrated as having H. heilmannii, four were positive for H. felis, three were positive for both H. felis and H. heilmannii, and seven for unclassified Helicobacter species [137]. There is a single report of Helicobacter organisms in cats with gastric lymphoma [138]. Of the cases with gastric lymphoma, 17 were identified with lymphoblastic lymphoma. In that study, histopathological samples from 47 cats with gastro-intestinal disease were assessed; 14 demonstrated gastritis, 31 lymphoma, and 2 were normal. It was found that ‘sick’ cats were more likely to host Helicobacter organisms. Furthermore, 13/17 cases of lymphoblastic lymphoma were positive for H. heilmannii by fluorescent in situ hybridisation [138].
3. Parasitic Infections
One of the first reports of neoplasia occurring in association with infection was the observation that bladder cancers were more prevalent in men with schistosomiasis [139]. Since this time, it has been demonstrated in humans that the helminth-associated diseases, schistosomiasis, opisthorchiasis, and clonorchiasis, are highly carcinogenic [140]. Furthermore, it has been shown that the protozoal organism, Trypanosoma cruzi, has a dual role of being both carcinogenic and anti-carcinogenic [140]. The malaria parasite, Plasmodium falciparum, is strongly associated with Burkitt lymphoma in endemic areas, but only when co-infection with EBV is present [140].
One early report documented four cases of cholangiocarcinoma in cats parasitised by Platynosomum fastosum (P. fastosum) [141]. However, despite numerous reports of biliary tract infection by this trematode, there were no further reports of neoplasia until 2012, when post mortems of 3 of 11 cats infected with P. fastosum were reported to have cholangiocarcinoma [142]. In addition, there is a single case report of a cat with biliary cystadenoma associated with Opisthorchis viverrini infection [143], and, to date, there are no reports of Opisthorchis felineus leading to biliary neoplasia in the cat, despite reports in many other species [144][145][146].
4. Conclusion
This entry is adapted from the peer-reviewed paper 10.3390/vetsci9090467
References
- Beatty, J. Viral causes of feline lymphoma: Retroviruses and beyond. Vet. J. 2014, 201, 174–180.
- Rezanka, L.J.; Rojko, J.L.; Neil, J.C. Feline leukemia virus: Pathogenesis of neoplastic disease. Cancer Investig. 1992, 10, 371–389.
- Shelton, G.H.; Grant, C.K.; Cotter, S.M.; Gardner, M.B.; Hardy, W.D., Jr.; DiGiacomo, R.F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: A retrospective study (1968–1988). J. Acquir. Immunedeficiency Syndr. 1990, 3, 623–630.
- Hartmann, K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet. Immunol. Immunopathol. 2011, 143, 190–201.
- Lutz, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline leukaemia. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 565–574.
- Willett, B.J.; Hosie, M.J. Feline Leukaemia virus: Half a century since its discovery. Vet. J. 2013, 195, 16–23.
- Chiu, E.S.; Hoover, E.A.; VandeWoude, S. A retrospective examination of Feline Leukemia subgroup characterization: Viral interference assays to deep sequencing. Viruses 2018, 10, 10010029.
- Arico, D.; Drago, V.; Foster, P.S.; Heilman, K.M.; Williamson, J.; Ferri, R. Effects of NREM sleep instability on cognitive processing. Sleep Med. 2010, 11, 791–798.
- Jarrett, O.; Hardy, W.D., Jr.; Golder, M.C.; Hay, D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int. J. Cancer 1978, 21, 334–337.
- Jarrett, O.; Russel, P.H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int. J. Cancer 1978, 21, 466–472.
- Sarma, P.S.; Log, T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology 1973, 54, 160–169.
- Sarma, P.S.; Log, T. Viral interference in feline leukemia-sarcoma complex. Virology 1971, 44, 352–358.
- Stewart, H.; Jarrett, O.; Hosie, M.J.; Willett, B.J. Are endogenous feline leukemia viruses really endogenous? Vet. Immunol. Immunopathol. 2011, 143, 325–331.
- Stewart, M.A.; Warnock, M.; Wheeler, A.; Wilkie, N.; Mullins, J.I.; Onions, D.E.; Neil, J.C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence of the recombinational origin of subgroup B viruses. J. Virol. 1986, 58, 825–834.
- Roy-Burman, P. Endogenous env elements: Partners in generation of pathogenic feline leukemia viruses. Virus Genes 1996, 1996, 147–161.
- Hoover, E.A.; Kociba, G.J.; Hardy, W.D., Jr.; Yohn, D.S. Erythroid hypoplasia in cats inoculated with feline leukemia virus. J. Natl. Cancer Inst. 1974, 53, 1271–1276.
- Mackey, L.; Jarrett, W.; Jarrett, O.; Laird, H. Anemia associated with feline leukemia virus infection in cats. J. Natl. Cancer Inst. 1975, 54, 209–217.
- Tury, S.; Giovannini, D.; Ivanova, S.; Touhami, J.; Courgnaud, V.; Battini, J.L. Identification of Copper Transporter 1 as a Receptor for Feline Endogenous Retrovirus ERV-DC14. J. Virol. 2022, 96, e0022922.
- Anai, Y.; Ochi, H.; Watanabe, S.; Nakagawa, S.; Kawamura, M.; Gojobori, T.; Nishigaki, K. Infectious endogenous retroviruses in cats and emergence of recombinant viruses. J. Virol. 2012, 86, 8634–8644.
- Miyake, A.; Kawasaki, J.; Ngo, H.; Makundi, I.; Muto, Y.; Khan, A.H.; Smith, D.J.; Nishigaki, K. Reduced Folate Carrier: An Entry Receptor for a Novel Feline Leukemia Virus Variant. J. Virol. 2019, 93, e00269-19.
- Miyake, A.; Watanabe, S.; Hiratsuka, T.; Ito, J.; Ngo, M.H.; Makundi, I.; Kawasaki, J.; Endo, Y.; Tsujimoto, H.; Nishigaki, K. Novel Feline Leukemia Virus Interference Group Based on the env Gene. J. Virol. 2016, 90, 4832–4837.
- Donahue, P.R.; Quackenbush, S.L.; Gallo, M.V.; deNoronha, C.M.C.; Overbaught, J.; Hoover, E.A.; Mullins, J.I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J. Virol. 1991, 65, 4461–4469.
- Mullins, J.I.; Hoover, E.A.; Overbaugh, J.; Quackenbush, S.L.; Donahue, P.R.; Poss, M.L. FeLV-FAIDS induced immunodeficiency syndrome in cats. Vet. Immunol. Immunopathol. 1989, 21, 25–37.
- Chiu, E.S.; VandeWoude, S. Presence of Endogenous Viral Elements Negatively Correlates with Feline Leukemia Virus Susceptibility in Puma and Domestic Cat Cells. J. Virol. 2020, 94, e01274-20.
- Chiu, E.S.; McDonald, C.A.; VandeWoude, S. Endogenous Feline Leukemia Virus (FeLV) siRNA transcription may interfere with exogenous FeLV infection. J. Virol. 2021, 95, e00070-00021.
- Hartmann, K. Feline leukemia virus infection. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St Louis, MO, USA, 2012; pp. 108–136.
- Pacitti, A.M.; Jarrett, O.; Hay, D. Transmission of feline leukaemia virus in the milk of a non-viraemic cat. Vet. Rec. 1986, 118, 381–384.
- Rojko, J.L.; Hoover, E.A.; Quackenbush, S.L.; Olsen, R.G. Reactivation of latent feline leukaemia virus infection. Nature 1982, 298, 385–388.
- Fujino, Y.; Ohno, K.; Tsujimoto, H. Molecular pathogensis of feline leukemia virus-induced malignancies: Insertional mutagenesis. Vet. Immunol. Immunopathol. 2008, 123, 138–143.
- Fujino, Y.; Liao, C.-P.; Zhao, Y.-S.; Pan, J.; Mathes, L.E.; Hayes, K.A.; Ohno, K.; Tsujimoto, H.; Roy-Burman, P. Identification of a novel common proviral integration site, flit-1, in feline leukemia virus induced thymic lymphoma. Virology 2009, 386, 16–22.
- Hardy, W.D., Jr. The Feline Sarcoma Viruses. J. Am. Anim. Hosp. Assoc. 1981, 17, 981–997.
- Bonham, L.; Lobellerich, P.A.; Henderson, L.A.; Levy, L.S. Transforming potential of a myc-containing variant of feline leukemia-virus in vitro in early passage feline cells. J. Virol. 1987, 61, 3072–3081.
- Rohn, J.L.; Linenberger, M.L.; Hoover, E.A.; Overbaugh, J. Evolution of Feline Leukemia Virus variant genomes with insertions, deletions, and defective envelope genes in infected cats with tumours. J. Virol. 1994, 68, 2458–2467.
- Forman, L.W.; Pal-Ghosh, R.; Spanjaard, R.A.; Faller, D.V.; Ghosh, S.K. Identification of LTR-specific small non-coding RNA in FeLV infected cells. FEBS Lett. 2009, 583, 1386–1390.
- Forrest, D.; Onions, D.E.; Lees, G.; Neil, J.C. Altered structure and expression of c-myc in feline T-cell tumours. Virology 1987, 158, 194–205.
- Miura, T.; Shibuya, M.; Tsujimoto, H.; Fukasawa, M.; Hayami, M. Molecular cloning of a feline leukemia provirus adjacent to the c-myc gene in a feline T-cell leukemia cell line and the unique structure of its long terminal repeat. Virology 1989, 169, 458–461.
- Miura, T.; Tsujimoto, H.; Fukasawa, M.; Kodama, T.; Shibuya, M.; Hasegawa, A.; Hayami, M. Structural abnormality and over-expression of the myc gene in feline leukemias. Int. J. Cancer 1987, 40, 564–569.
- Levy, L.S.; Lobelle-Rich, P.A.; Overbaugh, J.; Abkowitz, J.L.; Fulton, R.; Roy-Burman, P. Coincident involvement of flvi-2, c-myc, and novel env genes in natural and experimental lymphosarcomas induced by feline leukemia virus. Virology 1993, 196, 892–895.
- Tsatanis, C.; Fulton, R.; Nishigaki, K.; Tsujimoto, H.; Levy, L.S.; Terry, A.; Speandidos, D.; Onions, D.E.; Neil, J.C. Genetic determinants of feline leukemia virsu-induced lymphoid tumors: Patterns of proviral insertion and gene rearrangement. J. Virol. 1994, 68, 8296–8303.
- Neil, J.C.; Hughes, D.; McFarlane, R.; Wilkie, N.M.; Onions, D.E.; Lees, G.; Jarrett, O. Transduction and rearrangement of the myc gene by feline leukaemia virus innnaturally occurring T-cell leukaemias. Nature 1984, 308, 814–820.
- Levy, L.S.; Gardner, M.B.; Casey, J.W. Isolation of a feline leukaemia provirus containing the oncogene myc from a feline lymphosarcoma. Nature 1984, 308, 853–856.
- Bolin, L.L.; Levy, L.S. Viral determinants of FeLV infection and pathogenesis: Lessons learned from analysis of an natural cohort. Viruses 2011, 3, 1681–1698.
- Levy, L.S.; Lobelle-Rich, P.A.; Overbaugh, J. flvi-2, a target of retroviral insertional mutagenesis in feline thymic lymphosarcoma, encodes bmi-1. Oncogene 1993, 8, 1833–1838.
- Levy, L.S.; Lobelle-Rich, P.A. Insertional mutagenesis of flvi-2 in tumors induced by infection with LC-FeLV, a myc-containing strain of feline leukemia virus. J. Virol. 1992, 66, 2885–2892.
- Tsujimoto, H.; Fulton, R.; Nishigaki, K.; Matsumoto, Y.; Hasegawa, A.; Tsujimoto, A.; Cevario, S.; O’Brien, S.J.; Terry, A.; Onions, D.E.; et al. A common proviral integration region, fit-1, in T-cell tumors induced by myc-containing feline leukemia viruses. Virology 1993, 196, 845–848.
- Levesque, K.S.; Bonham, L.; Levy, L.S. flvi-1, a common integration domain of feline leukemia virus in naturally occurring lymphomas of a particular type. J. Virol. 1990, 64, 3455–3462.
- Attisano, L.; Carcamo, J.; Ventura, F.; Weis, F.M.; Massague, J.; Wrana, J.L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993, 75, 671–680.
- ten Dijke, P.; Ichijo, H.; Franzen, P.; Schulz, P.; Saras, J.; Toyoshima, H.; Heldin, C.H.; Miyazono, K. Activin receptor-like kinases: A novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 1993, 8, 2879–2887.
- Francis, D.P.; Essex, M.; Cotter, S.M.; Gutensohn, N.; Jakowski, R.; Hardy, W.D., Jr. Epidemiological association between virus-negative feline leukemia and the horizontally trasmitted feline-leukemia virus. Cancer Res. 1981, 39, 3866–3870.
- Hardy, W.D., Jr.; McClelland, A.J.; Zuckerman, E.E.; Snyder, H.W., Jr.; MacEwen, E.G.; Francis, D.; Essex, M. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLV. Nature 1980, 288, 90–92.
- Rojko, J.L.; Kociba, G.J.; Abkowitz, J.L.; Hamilton, K.L.; Hardy, W.D., Jr.; Ihle, J.N.; O’Brien, S.J. Feline lymphomas-immunological and cytochemical characterization. Cancer Res. 1989, 49, 345–351.
- Fulton, R.; Forrest, D.; McFarlane, R.; Onions, D.E.; Neil, J.C. Retroviral transduction of T-cell antigen receptor b-chain and myc genes. Nature 1987, 326, 190–194.
- Beatty, J.A.; Tasker, S.; Jarrett, O.; Lam, A.; Gibson, S.; Noe-Nordberg, A.; Phillips, A.; Fawcett, A.; Barrs, V.R. Marker of feline leukaemia virus infection or exposure in cats from a region of low seroprevalence. J. Feline Med. Surg. 2011, 13, 927–933.
- Stützer, B.; Simon, K.; Lutz, H.; Majzoub, M.; Hermanns, W.; Hirschberger, J.; Sauter-Louis, C.; Hartmann, K. Incidence of persistent viraemia and latent feline leukaemia virus infection in cats with lymphoma. J. Feline Med. Surg. 2011, 13, 81–87.
- Herring, E.S.; Troy, G.C.; Toth, T.E.; Forrester, S.D.; Weigt, L.A.; Herring, I.P. Detection of feline leukaemia vius in blood and bone marrow of cats with carying suspicion of latent infection. J. Feline Med. Surg. 2001, 3, 133–141.
- Gabor, L.J.; Jackson, M.L.; Trask, B.; Malik, R.; Canfield, P.J. Feline leukaemia virus status of Australian cats with lymphosarcoma. Aust. Vet. J. 2001, 79, 476–481.
- Jackson, M.L.; Haines, D.M.; Meric, S.M.; Misra, V. Feline leukemia virus detection by immunohistochemistry and polymerase chain-reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma. Can. J. Vet. Res. Rev. Can. Rech. Vet. 1993, 57, 269–276.
- Weiss, A.T.A.; Klopfleisch, R.; Gruber, A.D. Prevalence of feline leukaemia provirus DNA in feline lymphomas. J. Feline Med. Surg. 2010, 12, 929–935.
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a T-lymphotrophic virus from domestic cats with an immunodeficiency like syndrome. Science 1987, 235, 790–793.
- Sellon, R.K.; Hartmann, K. Feline immunodeficiency virus infection. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier Saunders: St Louis, MO, USA, 2012; pp. 136–149.
- Hosie, M.J.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Lloret, A.; Lutz, H.; et al. Feline Immunodeficiency. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 575–584.
- Liem, B.P.; Dhand, N.K.; Pepper, A.E.; Barrs, V.R.; Beatty, J. Clinical features and survival in cats naturally infected with feline immunodeficiency virus. J. Vet. Intern. Med. 2013, 27, 798–805.
- Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Feline infectious peritonitis. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 594–604.
- Magden, E.; Quackenbush, S.L.; VandeWoude, S. FIV associated neoplasms-A mini-review. Vet. Immunol. Immunopathol. 2011, 143, 227–234.
- Callanan, J.J.; Jones, B.A.; Irvine, J.; Willett, B.J.; McCandlish, I.A.P.; Jarrett, O. Histological classification and immunophenotype of lymphosarcoma in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 1996, 33, 264–272.
- Terry, A.; Callanan, J.J.; Fulton, R.; Jarrett, O.; Neil, J.C. Molecular analysis of tumours from feline immunodeficiency virus (FIV)-infected cats: An indirect role for FIV? Int. J. Cancer 1995, 61, 227–232.
- Gabor, L.J.; Love, D.N.; Malik, R.; Canfield, P.J. Feline Immunodeficiency virus status of Australian Cats with lymphosarcoma. Aust. Vet. J. 2001, 79, 540–545.
- Rosenburg, M.P.; Hohenhaus, A.E.; Matus, R.E. Monoclonal gammopathy and lymphoma in a cat infected with feline immunodeficiency virus. J. Am. Anim. Hosp. Assoc. 1991, 27, 335–337.
- Endo, Y.; Cho, K.W.; Nishigaki, K.; Monoi, Y.; Nischimura, Y.; Mizuno, T.; Goto, Y.; Watari, T.; Tsujimoto, H.; Hasegawa, A. Molecular characteristics of malignant lymphomas in cats naturally infected with feline immunodeficiency virus. Vet. Immunol. Immunopathol. 1997, 123, 153–167.
- Wang, J.; Kyaw-Tanner, M.; Lee, C.; Robinson, W.F. Characterization of lymphosarcomas in Australian cats using polymerase chain reaction and immunohistochemical examination. Aust. Vet. J. 2001, 79, 41–46.
- Ishida, T.; Washizu, T.; Toriyabe, K.; Motoyoshi, S.; Tomodo, I.; Pedersen, N.C. FIV infection in cats of Japan. J. Am. Vet. Med. Assoc. 1989, 194, 221–225.
- Hutson, C.; Rideout, B.A.; Pedersen, N.C. Neoplasia associated with Feline Immunodeficiency virus infection in cats of southern California. J. Am. Vet. Med. Assoc. 1991, 199, 1357–1362.
- Yamomoto, J.K.; Hansen, H.; Ho, E.W.; Morishita, T.Y.; Okuda, T.Y.; Sawa, T.R.; Nakamura, R.M.; Pedersen, N.C. Epidemiological and clincial aspects of feline immunodeficiency virus in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989, 194, 213–220.
- Alexander, R.; Robinson, W.F.; Mill, J.N.; Sherry, C.R.; Sherard, E.; Paterson, A.J.; Shaw, S.E.; Clark, W.T.; Hollingsworth, T. Isolation of feline immunodeficiency cirus from three cats with lymphoma. Aust. Vet. Pract. 1989, 19, 93–97.
- Sabine, M.; Michelsen, J.; Thomas, F.; Zheng, M. Feline AIDS. Aust. Vet. Pract. 1988, 18, 105–107.
- Hopper, C.D.; Sparkes, A.H.; Gruffydd-Jones, T.; Crispin, S.M.; Muir, P.; Harbour, D.A.; Stokes, C.R. Clinical and laboratory findings in cats infected with Feline Immunodeficiency Virus. Vet. Rec. 1989, 125, 321–346.
- Beatty, J.A.; Lawrence, C.E.; Callanan, J.J.; Grant, C.K.; Gault, E.A.; Neil, J.C.; Jarrett, O. Feline Immunodeficiency virus (FIV)-associated lymphoma: A potential role for immune dysfunction in tumourigenesis. Vet. Immunol. Immunopathol. 1998, 65, 309–322.
- Callanan, J.J.; McCandlish, I.A.P.; O’Neil, B.; Lawrence, C.E.; Rigby, M.; Pacitti, A.M.; Jarrett, O. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection. Vet. Rec. 1992, 130, 293–295.
- Poli, A.; Abramo, F.; Cavicchio, P.; Bandecchi, P.; Ghelardi, E.; Pistello, M. Malignant lymphoma associated with experimentally induced feline immunodeficiency virus infection. J. Comp. Pathol. 1994, 110, 319–328.
- Shelton, G.H.; Waltier, R.M.; Connor, S.C.; Grant, C.K. Prevalence of feline immunodeficiency virus and deline leukaemia virus infections in pet cats. J. Am. Anim. Hosp. Assoc. 1989, 25, 7–12.
- Rahman, R.; Gopinath, D.; Buajeeb, W.; Poomsawat, S.; Johnson, N.W. Potential role of Epstein-Barr virus in oral potentially malignant disorders and oral squamous cell carcinoma: A scoping review. Viruses 2022, 14, 801.
- Cesarman, E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011, 305, 163–174.
- Beatty, J.A.; Troyer, R.M.; Carver, S.; Barrs, V.R.; Espinasse, F.; Conradi, O.; Stutzman-Rodriguez, K.; Chan, C.C.; Tasker, S.; Lappin, M.R.; et al. Felis catus gammaherpesvirus 1; a widely endemic potential pathogen of domestic cats. Virology 2014, 460, 100–107.
- Troyer, R.M.; Beatty, J.A.; Stutzman-Rodriguez, K.R.; Carver, S.; Lozano, C.C.; Lee, J.S.; Lappin, M.R.; Riley, S.P.D.; Serieys, L.E.K.; Logan, K.A.; et al. Novel gammaherpesviruses in north American domestic cats, bobcats, and pumas: Identification, prevalence, and risk factors. J. Virol. 2014, 88, 3914–3924.
- Ertl, R.; Korb, M.; Langbein-Detsch, I.; Klein, D. Prevalence and risk factors of gammaherpesvirus infection in domestic cats in Central Europe. J. Virol. 2015, 12, 146.
- Kurissio, J.K.; Rodrigues, M.V.; Taniwaki, S.A.; de Souza Zanutto, M.; Filoni, C.; Galdino, M.V.; Araújo, A.P., Jr. Felis catus gammaherpesvirus 1 (FcaGHV1) and coinfections with feline viral pathogens in domestic cats in Brazil. Cienc. Rural. 2018, 48, e20170480.
- Makundi, I.; Koshida, Y.; Endo, Y.; Nishigaki, K. Identification of Felis catus Gammaherpesvirus 1 in Tsushima Leopard Cats (Prionailurus bengalensis euptilurus) on Tsushima Island, Japan. Viruses 2018, 1, 378.
- McLuckie, A.; Tasker, S.; Dhand, N.K.; Spencer, S.; Beatty, J.A. High prevalence of Felis catus gammaherpesvirus 1 infection in haemoplasma-infected cats supports co-transmission. Vet. J. 2016, 214, 117–121.
- Novacco, M.; Ranjbar Kohan, N.; Stirn, M.; Meli, M.L.; Díaz-Sánchez, A.; Boretti, F.S.; Hofmann-Lehmann, R. Prevalence, Geographic Distribution, Risk Factors and Co-Infections of Feline Gammaherpesvirus Infections in Domestic Cats in Switzerland. Viruses 2019, 11, 721.
- Powers, J.A.; Chiu, E.S.; Kraberger, S.J.; Roelke-Parker, M.; Lowery, I.; Erbeck, K.; Troyer, R.; Carver, S.; VandeWoude, S. Feline leukemia virus (FeLV) disease outcomes in a domestic cat breeding colony: Relationship to endogenous FeLV and other chronic viral infections. J. Virol. 2018, 92, e00649-18.
- Rose, E.C.; Tse, T.Y.; Oates, A.W.; Jackson, K.; Pfeiffer, S.; Donahoe, S.L.; Setyo, L.; Barrs, V.R.; Beatty, J.A.; Pesavento, P.A. Oropharyngeal shedding of Gammaherpesvirus DNA by Cats, and Natural Infection of Salivary Epithelium. Viruses 2022, 14, 566.
- Parisi, F.; Freer, G.; Mazzanti, C.M.; Pistello, M.; Poli, A. Mouse Mammary Tumor Virus (MMTV) and MMTV-like Viruses: An In-depth Look at a Controversial Issue. Viruses 2022, 14, 977.
- Hsu, W.-L.; Lin, H.-Y.; Chiou, S.-S.; Chang, C.-C.; Wang, S.-P.; Lin, K.-H.; Chulakasian, S.; Wong, M.-L.; Chang, S.-C. Mouse mammary tumor virus-like nucleotide sequences in canine and feline mammary tumors. J. Clin. Microbiol. 2010, 48, 4352–4362.
- Callahan, R.; Smith, G.H. MMTV-induced mammary tumorigenesis: Gene discovery, progression to malignancy and cellular pathways. Oncogene 2000, 19, 992–1001.
- Parisi, F.; Muscatello, L.V.; Civita, P.; Lessi, F.; Menicagli, M.; Millanta, F.; Brunetti, B.; Benazzi, C.; Sarli, G.; Freer, G.; et al. Pathological Features and Molecular Phenotype of MMTV Like-Positive Feline Mammary Carcinomas. Animals 2021, 11, 2821.
- Amber, K.; McLeod, M.P.; Nouri, K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol. Surg. 2013, 39, 232–238.
- Munday, J.S.; Thomson, N.A. Papillomaviruses in Domestic Cats. Viruses 2021, 13, 1664.
- Munday, J.S. Papillomaviruses in felids. Vet. J. 2014, 199, 340–347.
- Munday, J.S.; Sharp, C.R.; Beatty, J. Novel Virus: Update on the significance of papillomavirus infections in cats. J. Feline Med. Surg. 2019, 21, 409–418.
- Munday, J.S.; Kiupel, M. Papillomavirus-Associated Cutaneous Neoplasia in Mammals. Vet. Pathol. 2010, 47, 254–264.
- Ito, S.; Chambers, J.K.; Mori, C.; Sumi, A.; Omachi, T.; Nakayama, H.; Uchida, K. Comparitive In Vitro and In Vivo studies on Feline, Canine, and Human Merkel Cell Carcinoma. Vet. Pathol. 2021, 58, 276–287.
- Ito, S.; Chambers, J.K.; Sumi, A.; Yamashita-Kawanishi, N.; Omachi, T.; Haga, T.; Nakayama, H.; Uchida, K. Involvement of Felis catus papillomavirus type 2 in the tumorigenesis of feline Merkel cell carcinoma. Vet. Pathol. 2022, 59, 63–74.
- Egberink, H.; Thiry, E.; Mostl, K.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hosie, M.; Hartmann, K.; et al. Feline Viral Papillomatosis: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 560–562.
- Munday, J.S.; French, A.F.; Peters-Kennedy, J.; Orbell, G.M.B.; Gwynne, K. Increased p16CDKN2A Protein Within Feline Cutaneous Viral Plaques, Bowenoid In Situ Carcinomas, and a Subset of Invasive Squamous Cell Carcinoma. Vet. Pathol. 2011, 48, 460–465.
- Vascellari, M.; Mazzei, M.; Zanardello, C.; Melchiotti, E.; Albanese, F.; Forzan, M.; Abramo, F. Felis catus Papillomavirus Types 1, 2, 3, 4, and 5 in Feline Bowenoid in Situ Carcinoma: An In Situ Hybridization Study. Vet. Pathol. 2019, 56, 818–825.
- Demos, L.E.; Munday, J.S.; Lange, C.E.; Bennett, M.D. Use of fluorescence in situ hybridization to detect Felis catus papullomavirus in feline Bowenoid in situ carcinoma. J. Feline Med. Surg. 2019, 21, 575–580.
- Wilhelm, S.; Degorce-Rubiales, F.; Godson, D.; Favrot, C. Clinical, histological and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Vet. Dermatol. 2006, 17, 424–431.
- Kok, M.K.; Yamashita-Kawanishi, N.; Chambers, J.K.; Haritani, M.; Ushigusa, T.; Haga, T.; Nakayama, H.; Uchida, K. Pathologica characterization of Felis catus papillomavirus type 5 (FcaPV-5)-associated viral plaques and Bowenoid in situ carcinoma in a Domestic Shorthair cat. J. Vet. Med. Sci. 2019, 81, 660–666.
- Lange, C.E.; Tobler, K.; Markau, T.; Alhaidari, Z.; Bornand, V.; Stöcklif, R.; Trüsselg, M.; Ackermannb, M.; Favrota, C. Sequence and classification of FdPV2, a papillomavirus isolated from feline Bowenoid in situ carcinomas. Vet. Microbiol. 2009, 137, 60–65.
- Munday, J.S.; Benfell, M.W.; French, A.F.; Orbell, G.M.B.; Thomson, N.A. Bowenoid in situ carcinomas in two Devon Rex cats: Evidence of unusually aggressive neoplasm behaviour in this breed and detection of papillomaviral gene expression in primary and metastatic lesions. Vet. Dermatol. 2016, 27, 215-e55.
- Munday, J.S.; Fairley, R.; Atkinson, K. The detection of Felis catus papillomavirus 3 DNA in a feline bowenoid in situ carcinoma with novel histologic features and benign clinical behavior. J. Vet. Diagn. Investig. 2016, 28, 612–615.
- Favrot, C.; Welle, M.; Heimann, M.; Godson, D.L.; Gusdetti, F. Clinical, histologic, and immunohistochemical analyses of feline squamous cell carcinoma in situ. Vet. Pathol. 2009, 46, 25–33.
- Munday, J.S.; Kiupel, M.; French, A.F.; Howe, L. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet. Dermatol. 2008, 19, 259–263.
- Thomson, N.A.; Munday, J.S.; Dittmer, K.E. Frequent detection of transcriptionally active Felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. J. Gen. Virol. 2016, 97, 1189–1197.
- Altamura, G.; Power, K.; Martano, M.; degli Uberti, B.; Galiero, G.; De Luca, G.; Maiolino, P.; Borzacchiello, G. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Nature 2018, 8, 17529.
- Altamura, G.; Cardeti, G.; Cersini, A.; Eleni, C.; Cocumelli, C.; del Pino, L.E.B.; Razzuoli, E.; Martano, M.; Maiolino, P.; Borzacchiello, G. Detection of Felis catus papillomavirus type-2 DNA and viral gene expression suggest active infection in feline oral squamous cell carcinoma. Vet. Comp. Oncol. 2020, 18, 494–501.
- Altamura, G.; Corteggio, A.; Pacini, L.; Conte, A.; Pierantoni, G.M.; Tommasino, M.; Accardi, R.; Borzacchiello, G. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 2016, 496, 1–8.
- Munday, J.S.; Gibson, I.; French, A.F. Papillomaviral DNA and increased p16CDKN2A protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Vet. Dermatol. 2011, 22, 360–366.
- Munday, J.S.; French, A.F.; Thomson, N. Detection of DNA sequences from a novel papillomavirus in a feline basal cell carcinoma. Vet. Dermatol. 2017, 28, 236-e60.
- Munday, J.S.; Thomson, N.A.; Henderson, G.; Fairley, R.; Orbell, G.M. Identification of Felis catus papillomavirus 3 in skin neoplasms from four cats. J. Vet. Diagn. Investig. 2018, 30, 324–328.
- Teifke, J.P.; Kidney, B.A.; Löhr, C.V.; Yager, J.A. Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Vet. Dermatol. 2003, 14, 47–56.
- Munday, J.S.; Gedye, K.; Daudt, C.; Da Silva, F.C. The development of novel primer sets to specifically amplify each of the five different Deltapapillomaviruses that cause neoplasia after cross-species infection. Vet. Sci. 2021, 8, 208.
- Munday, J.S.; Thomson, N.; Dunowska, M.; Knight, C.G.; Laurie, R.E.; Hills, S. Genomic characterisation of the feline sarcoid-assoicated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet. Microbiol. 2015, 177, 289–295.
- Munday, J.S.; Knight, C.G.; Howe, L. The same papillomavirus is present in feline sarcoids from North America and New Zealand but not in any non-sarcoid feline samples. J. Vet. Diagn. Investig. 2010, 22, 97–100.
- Sundberg, J.P.; Van Ranst, M.; Montali, R.; Homer, B.L.; Miller, W.H.; Rowland, P.H.; Scott, D.W.; England, J.J.; Dunstan, R.W.; Mikaelian, I.; et al. Feline papillomas and papillomaviruses. Vet. Pathol. 2000, 37, 1–10.
- Munday, J.S.; Fairley, R.A.; MIlls, H.; Kiupel, M.; Vaatstra, B.L. Oral papillomas associated with Felis catus papillomavirus type 1 in 2 domestic cats. Vet. Pathol. 2015, 52, 1187–1190.
- Munday, J.S.; Howe, L.; French, A.; Squires, R.A.; Sugiarto, H. Detection of papillomaviral DNA sequences in a feline oral squamous cell carcinoma. Res. Vet. Sci. 2009, 86, 359–361.
- O’Neill, S.H.; Newkirk, K.M.; Anis, E.A.; Brahmbhatt, R.; Frank, L.A.; Kania, S.A. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet. Dermatol. 2011, 22, 68–74.
- Yamashita-Kawanishi, N.; Sawanobori, R.; Matsumiya, K.; Uema, A.; Chambers, J.K.; Uchida, K.; Shimakura, H.; Tsuzuki, M.; Chang, C.Y.; Chang, H.W.; et al. Detection of felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. J. Vet. Med. Sci. 2018, 80, 1236–1240.
- Munday, J.S.; Knight, C.G.; French, A.F. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res. Vet. Sci. 2011, 90, 280–283.
- Munday, J.S.; French, A.F. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Res. Vet. Sci. 2015, 100, 200–222.
- Rizzo, G.E.M.; Cabibbo, G.; Craxi, A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses 2022, 14, 986.
- Aghazadeh, M.; Shi, M.; Barrs, V.R.; McLuckie, A.J.; Lindsay, S.A.; Jameson, B.; Hampson, B.; Holmes, E.C.; Beatty, J.A. A novel hepadnavirus identified in an immunocompromised domestic cat in Australia. Viruses 2018, 10, 269.
- Lanave, G.; Capozza, P.; Diakoudi, G.; Catella, C.; Catucci, L.; Ghergo, P.; Stasi, F.; Barrs, V.; Beatty, J.; DeCaro, N.; et al. Identification of hepadnavirus in the sera of cats. Sci. Rep. 2019, 9, 10668.
- Pesavento, P.A.; Jackson, K.; Scase, T.; Tse, T.; Hampson, B.; Munday, J.S.; Barrs, V.R.; Beatty, J. A novel Hepadnavirus is associated with chrinic heaptitis and heparocellular carcinoma in cats. Viruses 2019, 11, 969.
- Aghazadeh, M.; Shi, M.; Pesavento, P.A.; Durham, A.C.; Polley, T.; Donahoe, S.L.; Troyer, R.M.; Barrs, V.R.; Holmes, E.C.; Beatty, J.A. Transcriptome Analysis and In Situ Hybridization for FcaGHV1 in Feline Lymphoma. Viruses 2018, 10, 464.
- Strauss-Ayali, D.; Scanziani, E.; Deng, D.; Simpson, K.W. Helicobacter spp. infection in cats: Evaluation of the humoural immune response and prevalence of gastric Helicobacter spp. Vet. Microbiol. 2001, 79, 253–265.
- Bridgeford, E.C.; Marini, R.P.; Feng, Y.; Parry, N.M.A.; Rickman, B.; Fox, J.G. Gastric Helicobacter species as a cause of feline gastric lymphoma: A viable hypothesis. Vet. Immunol. Immunopathol. 2008, 123, 106–113.
- Ferguson, A.R. Associated bilharziosis and primary malignant disease of the urinary bladder, with observations on a series of forty cases. J. Pathol. 1911, 16, 76–94.
- van Tong, H.; Brindley, P.J.; Meyer, C.G.; Velavan, T.P. Parasite Infection, Carcinogenesis and Human Malignancy. EBioMedicine 2017, 15, 12–23.
- Santos, J.A.; Lopes, M.A.F.; Schott, A.C.; Santos, A.E.S.; Porfirio, L.C.; Passos, L. Colangiocarcinomas em gatos com parasitismo de ductos biliares por Platynosomum fastosum. Pesqui. Vet. Bras. 1981, 1, 31–36.
- Andrade, R.L.F.S.; Dantas, A.F.M.; Galiza, G.J.N.; Carvalho, F.K.L. Platynosomum fastosum-induced cholangiocarcinomas in cats. Vet. Parasitol. 2012, 190, 277–280.
- Tiwananthagorn, S.; Srivorakul, S.; Khochakul, V. Biliary cystadenoma associated with Opisthorchis viverrini infection in a domestic cat (Felis catus). Vet. Parasitol. 2018, 258, 138–141.
- Gouveia, M.J.; Pakharukova, M.Y.; Laha, T.; Sripa, B.; Maksimova, G.A.; Rinaldi, G.; Brindley, P.J.; Mordvinov, V.A.; Amaro, T.; Santos, L.L.; et al. Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 2017, 38, 929–937.
- Kaewpitoon, N.; Kaewpitoon, S.J.; Pengsaa, P.; Sripa, B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J. Gastroenterol. 2008, 14, 666–674.
- Pakharukova, M.Y.; Zaparina, O.G.; Kapushchak, Y.K.; Baginskaya, N.V.; Mordvinov, V.A. Opisthorchis felineus infection provokes time-dependent accumulation of oxidative hepatobiliary lesions in the injured hamster liver. PLoS ONE 2019, 14, e0216757.
