With the advances in biomaterials and the understanding of the tumor microenvironment, in situ stimulus-responsive hydrogels, also called in situ smart hydrogels, have been extensively investigated for local anticancer therapy due to their injectability, compatibility and responsiveness to various stimuli (pH, enzyme, heat, light, magnetic fields, electric fields etc.).
- in situ hydrogel
- stimuli responsive
- local regional therapy
1. Introduction
Smart hydrogels, also called stimulus-responsive hydrogels, have excellent injectability and great potential to enhance drug efficiency and reduce systemic adverse effects. Hydrogels with a three-dimensional (3D) crosslinked network maintain a moist environment at the application site, and prolong the drug release and avoid it spreading to other healthy areas [1]. Compared with conventional hydrogel systems, in situ smart hydrogel systems exhibit the ability to change their properties, such as phase transition, swelling or shrinking in response to endogenous or exogenous stimuli (e.g., pH, light, temperature, enzymes, electrical fields, magnetic fields, etc.), resulting in injectability and controlled drug release [2][3]. Injectable in situ smart hydrogels are characterized by biocompatibility, negligible cytotoxicity, an outstanding drug loading ratio and controlled drug release, and have been extensively studied for cancer therapy in recent years [4].
The characteristics, significance and impact factors of in situ smart hydrogels for local antitumor therapy are described as below (Figure 1).
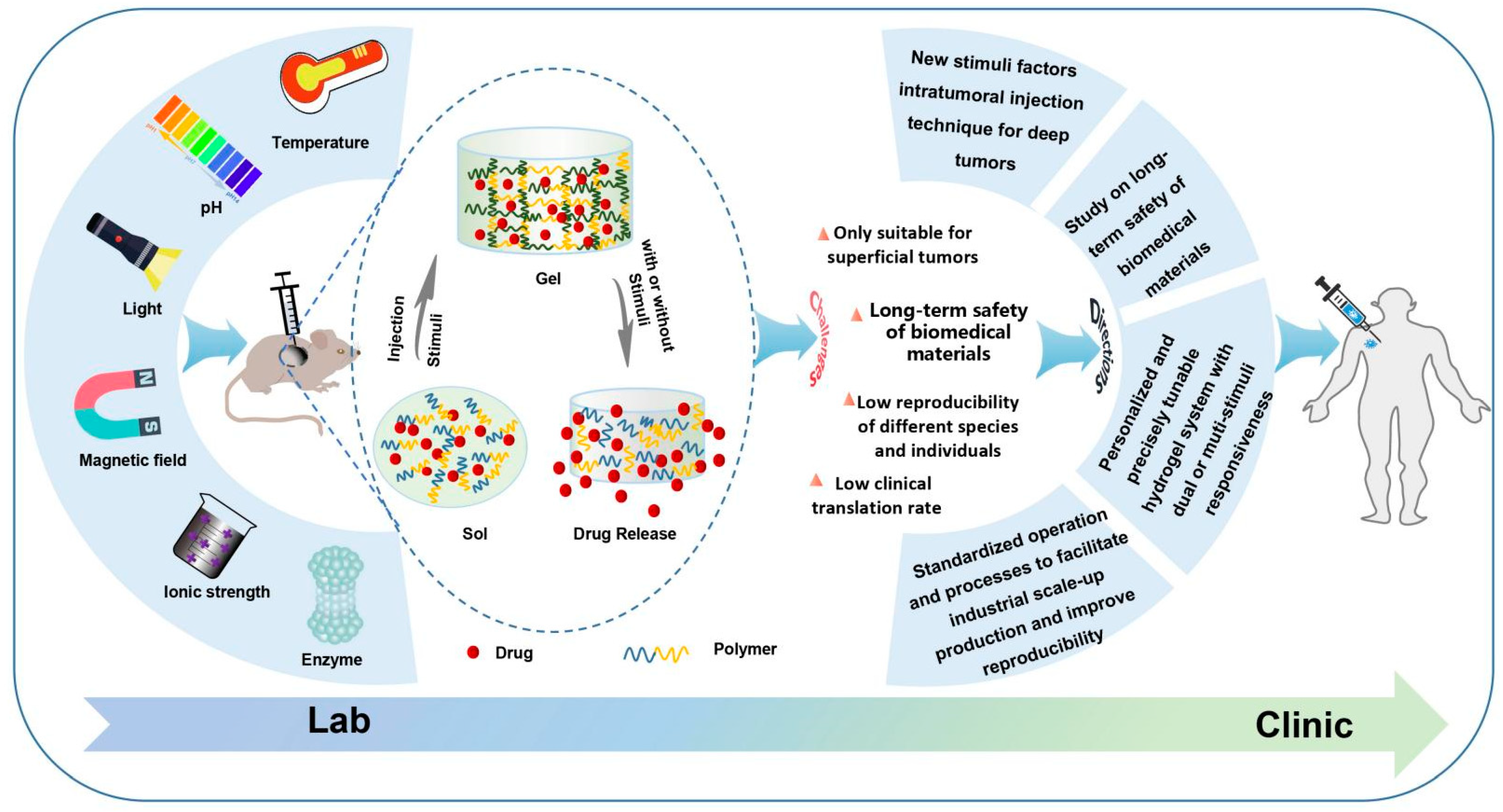
Figure 1. Schematic of the formation process and drug release, challenges and future directions of in situ smart hydrogels.
2. Characteristic of In Situ Smart Hydrogels for Local Administration
Intravenous drug delivery remains the dominant route of administration for cancer treatment. However, poor targeting of intravenous administration remains great challenges that induces low tumor accumulation and high systemic toxicities to healthy organs (Figure 2). In addition, long treatment cycles and repeated administration not only induce low compliance but also increase the economic pressure on patients. Comparing with systematic administration, drug biodistribution is localized to the tumor site by intratumoral administration, resulting in lower drug dose requirement and less systematic adverse effect and higher treatment efficacy (Figure 2). Nevertheless, the intratumoral injection of a drug solution may cause rapid diffusion, leading to a decreasing drug dose within the tumor and toxicity to the healthy tissues [5]. The 3D network structure of hydrogels allows antitumor drugs to be reserved in the hydrogels and to be continuously released from the hydrogels into the tumor site, which limits drug toxicity within a localized area. Moreover, the injectability, as a characteristic of in situ hydrogels, can be achieved by the sol–gel phase transition, which avoids painful surgical implantation and increases the compliance of patients. Generally, an ideal in situ smart hydrogel as a localized and continuous drug carrier is required to be characterized by biodegradation, low toxicity, good biocompatibility, suitable gel strength and gelation rate, and a rapid response to internal or external stimuli after injection [6][7].
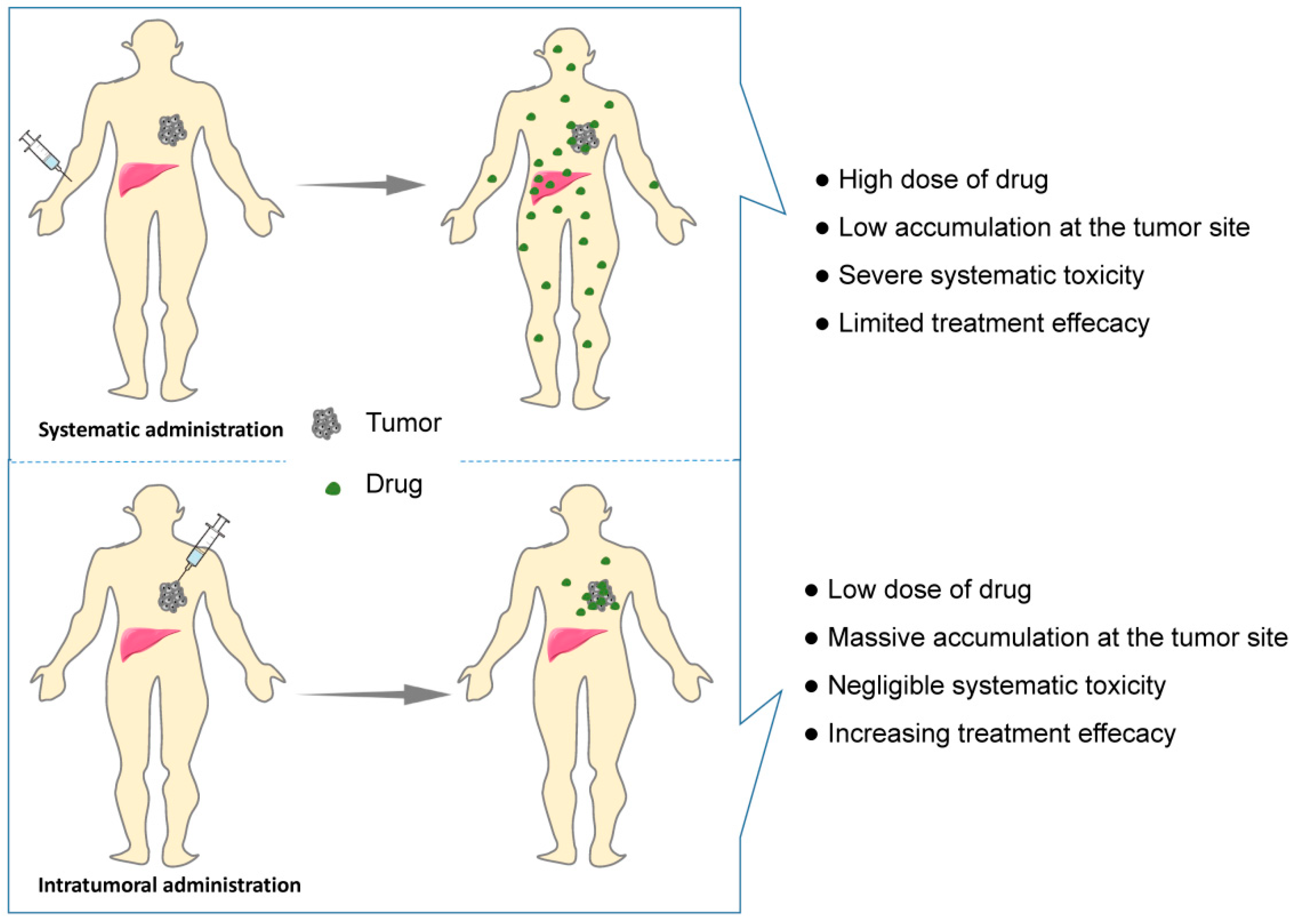
Figure 2. Schematic showing systematic vs. intratumoral administration of anticancer drugs.
3. Classification of Smart In Situ Hydrogels
Hydrogels can be classified by various parameters, such as the shape and size, the charge of the hydrogel-forming macromolecules and the mechanism of crosslinking during hydrogel formation [8][9]. Considering the ability to change the hydrogel properties in response to endogenous or exogenous stimuli, smart hydrogels can be divided into various smart hydrogels, such as temperature-, light-, pH-, enzyme- stimulus-responsive hydrogels (Figure 3). After the formation of drug-loading hydrogels with stimuli, the therapeutic agents can be released into the surrounding environment by diffusion via water-filled pores, implanted matrix, osmotic pumping or matrix erosion of the bulk and surface [10]. Intriguingly, the manner of drug release is well controlled in some kinds of smart hydrogels in response to various stimuli, such as enzyme, glutathione, and magnetic and electrical fields (Figure 4). The ability to react to the stimuli in the environment make smart hydrogels more appropriate candidates for tumor therapy systems.
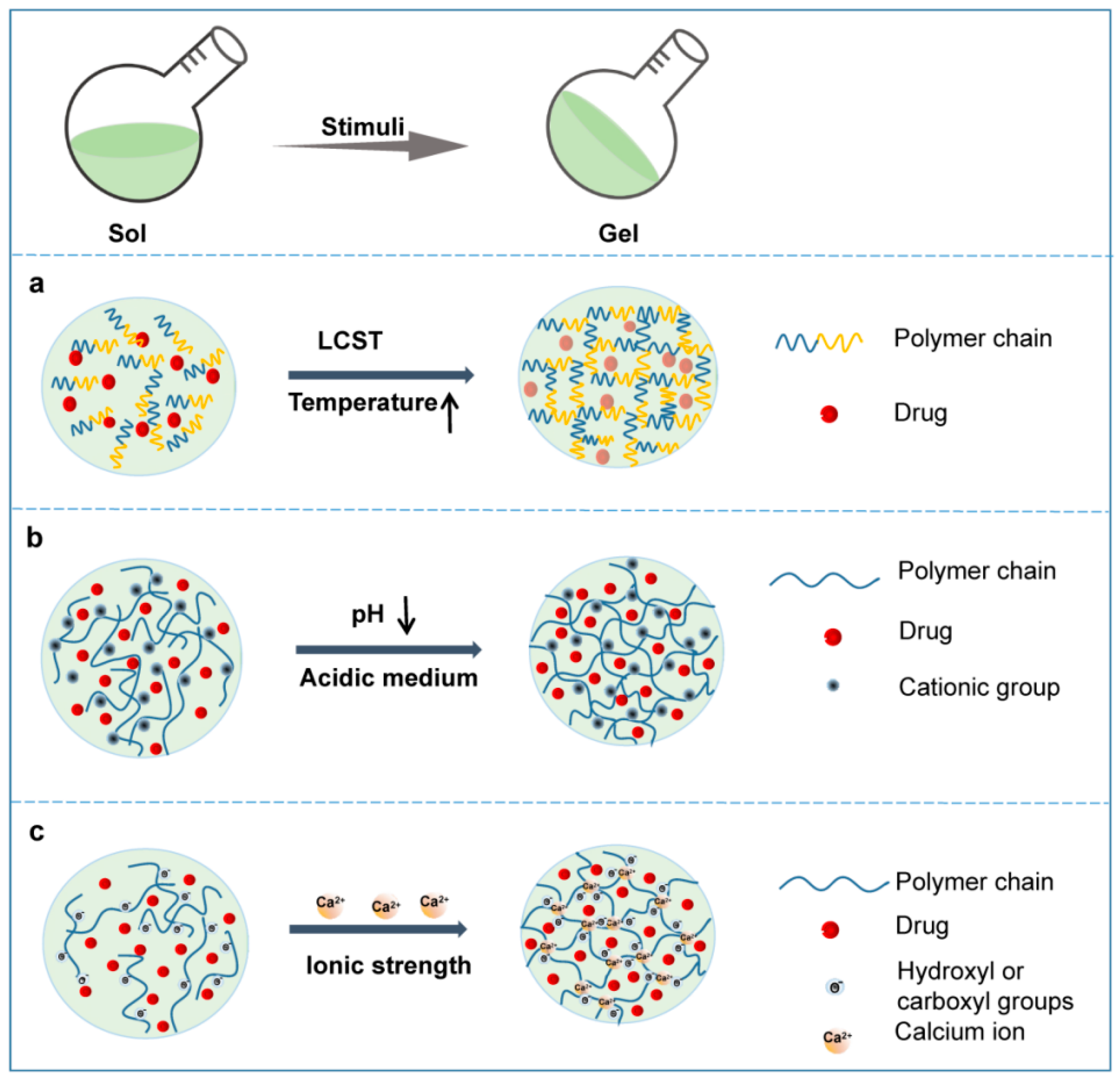
Figure 3. Schematic phase transition of representative hydrogels. (a) Temperature responsive hydrogel; (b) pH responsive hydrogel; (c) Ionic strength responsive hydrogel.
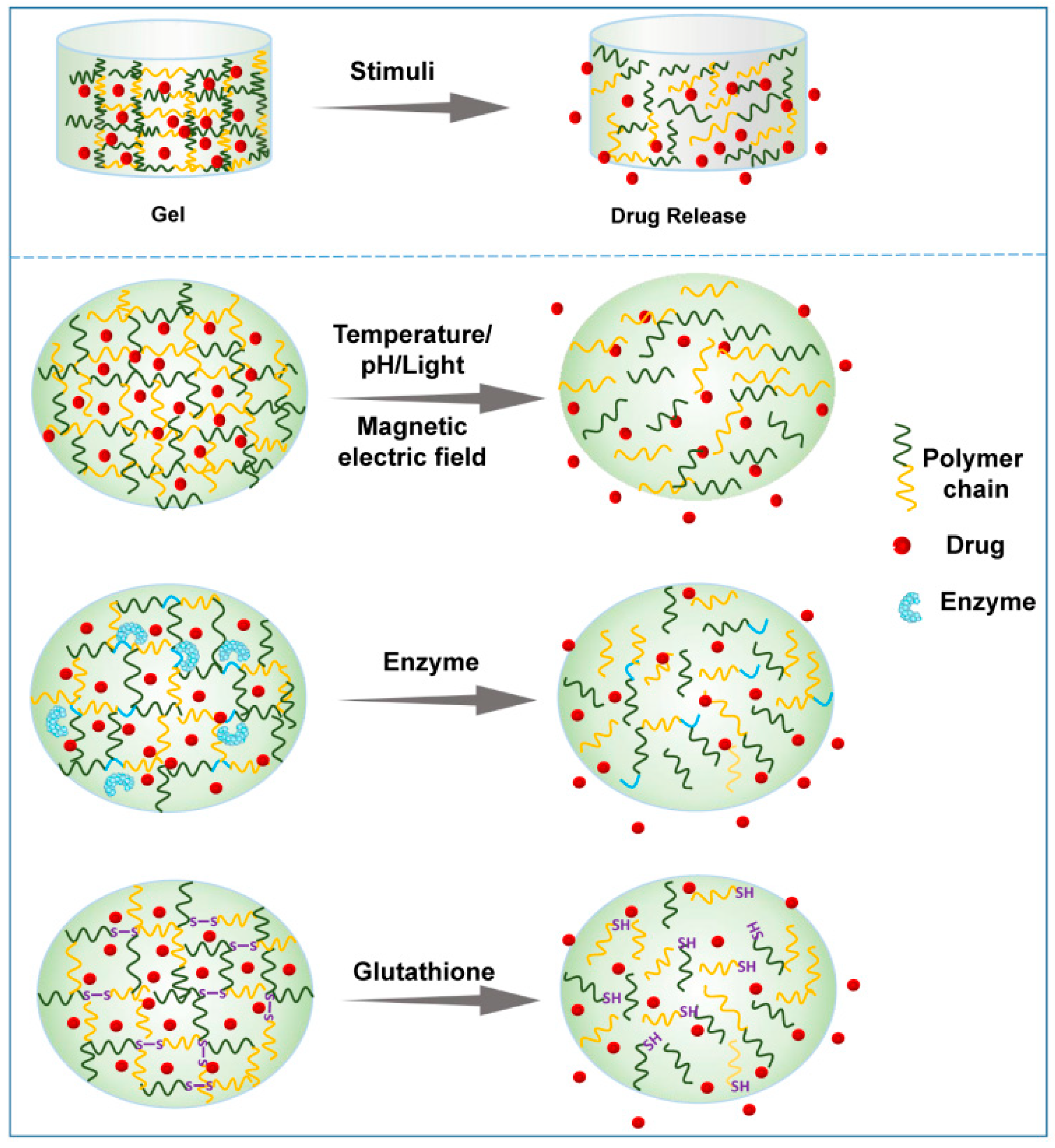
Figure 4. Schematic presentation of drug release from representative smart hydrogels upon various stimuli.
3.1. Temperature Responsiveness
Temperature-responsive hydrogels, which are capable of responding to temperature changes, have been extensively studied among the smart hydrogels [11][12]. As the temperature rises above or drops below a certain point, sol–gel phase transition of the thermoresponsive polymer solution occurs immediately and swelling or shrinking of the volume of the matrix happens simultaneously [1][13]. The temperature at which the phase transition proceeds is named the critical solution temperature (CST) [1]. According to the distinct phase transitions in response to temperature changes, thermoresponsive hydrogels are divided into lower CST (LCST) and upper CST (UCST) hydrogels. For LSCT hydrogels, the polymer matrices are miscible with water at a temperature lower than the LCST, but the solubility drops dramatically at or beyond the LCST, and then a sol–gel phase transition occurs (Figure 3a) [1]. In contrast to LCST systems, the phase transfer in UCST systems is reversed. Briefly, as the temperature rises, the solubility of the polymer matrix increases, and the system exhibits a gel-sol transition at the USCT. As the sol–gel phase transition occurs, the drugs in the system are reserved in the formed hydrogel concurrently and then continuously released from the hydrogel around the tumor site (Figure 4).
3.2. pH Responsiveness
The acidic tumor microenvironment is one of the main characteristics of almost all solid tumors due to the enhanced production of lactate by tumor cells resulting from their high rate of aerobic glycolysis [14]. Such acidification of the extracellular milieu and the increased alkalization of the cytoplasm of the tumor inspired the development of a pH-sensitive hydrogels to alleviate acidity within the TME back to the normal pH and suppress tumor growth (Figure 5). A pH-responsive hydrogel matrix typically contains a great number of weak acidic or basic groups, including amines, carboxylic acids, imines, etc., which are able to donate or accept protons. When the external pH changes, the properties of these polymers, such as solubility and volume, change due to the donation or acceptance of protons, resulting in phase transition and drug release. For example, after in situ injection, the amine groups of the polymer are positively charged (NH3+) by accepting protons because of the acidic external pH of the tumor microenvironment. The chains of the polymer will expand and swell as a result of the electrostatic repulsion between charges (Figure 3b) [15]. The enlarged mesh of swollen hydrogels facilitates drug diffusion through the network.
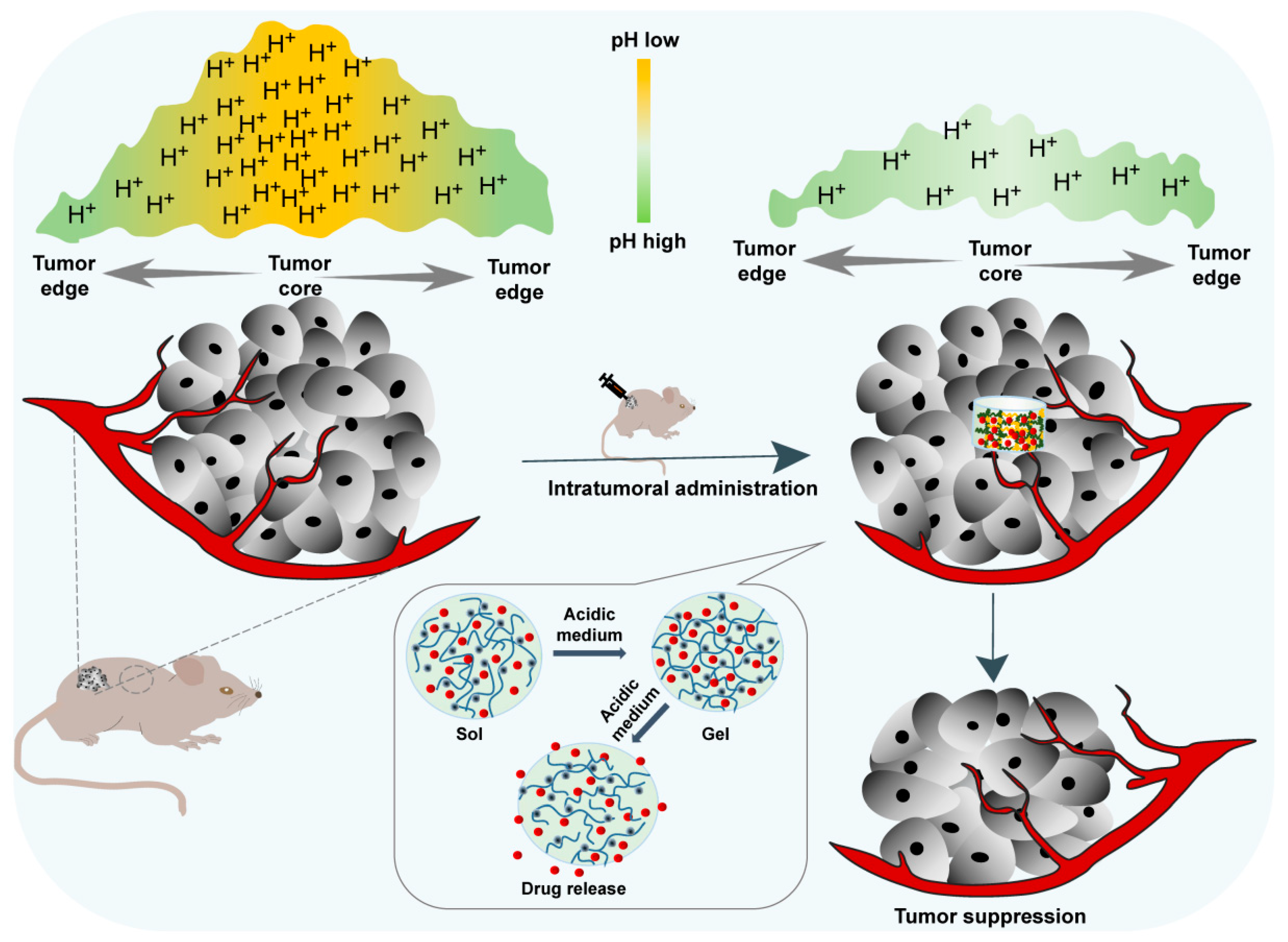
Figure 5. In situ pH-responsive hydrogel alleviates the tumor acidic microenvironment and enhances tumor growth inhibition.
3.3. Light Responsiveness
Light-responsive hydrogels undergo property changes and a phase transition as a consequence of light exposure, including near-infrared radiation (NIR), visible light and UV [16]. Accordingly, there are multiple strategies to prepare photosensitive hydrogels. Incorporation of a photosensitizer, most often photothermal agents, into the hydrogel system is a widely studied method. Photothermal agents generate heat through absorbing and emitting electromagnetic radiation, and then increase the temperature of the system, inducing the phase transition of thermoresponsive hydrogels. Another typical method of preparing light-responsive hydrogel is based on polymers containing a photoreactive moiety. The photoreactive moiety has the ability to respond to light through photochemical reactions, such as photoisomerization, crosslinking point photopolymerization and photocleavage, which lead to alterations in the hydrogel’s properties, such as the crosslinking density, charging state or hydrophilicity, ultimately inducing phase transition and drug release [17][18].
3.4. Magnetic-Field Responsiveness
Magnetic-field-sensitive hydrogels are usually composed of a 3D polymeric network and magnetic nanoparticles, most often iron oxide nanoparticles possessing paramagnetic properties [19][20][21]. These iron oxide nanoparticles can be vibrated through exposure to a magnetic field, resulting in a dramatic increase in the local temperature. Hence, magnetic-field-sensitive hydrogel systems generally combine thermosensitive properties with magnetic-field-responsive ability, in which the temperature increase induced by the magnetic field promotes sol–gel transition and drug release, thus facilitating the synergic efficacy of thermal and chemotherapeutic cytotoxicity. Two main advantages of magnetic-field-sensitive hydrogels are their excellent permeability to deep organs and low invasiveness, which have attracted special attention [22].
3.5. Ionic Strength Responsiveness
Ionic strength-sensitive in situ hydrogels undergo sol–gel phase transition via conformational changes in response to cations such as K+, Na+ and Ca2+ at the administration site. These hydrogels usually consist of ionizable and zwitterionic polymers such as alginate, deacetylated gellan gum, carboxymethyl dextran, polyacrylic acid and carboxybetaine derivatives [7]. Alginate is a typical material used in this kind of hydrogel, which contains abundant hydroxyl and carboxyl groups. When a certain solution of alginate is introduced to solutions containing monovalent (e.g., K+) or, more usually, divalent ions (e.g., Ca2+), the ions enter the discrete alginate and crosslink with the alginate polymer, achieving a rapid phase transition from a sol to a gel.
3.6. Enzyme Responsiveness
Enzymes, as signals to modulate metabolism, nutrition and energy conversion, are essential during tumorigenesis and tumor development. Importantly, the level of several enzymes in tumors significantly differs from that in normal tissues [23]. In an enzyme-responsive in situ hydrogel system, the matrix must contain a chemical moiety which needs to be an enzyme-specific substrate or a substrate mimicking and accessible to the enzymatic active center. The enzymatic reaction occurs after injection into the tumor site, and the biomaterial properties subsequently change, which leads to enzyme-mediated crosslinking or cleavage, inducing gelation or drug release (Figure 4) [24]. Notably, the high substrate specificity with regioselectivity and stereoselectivity are important characteristics of such enzyme-sensitive in situ hydrogels. Thus, it is necessary to further explore the specificity of enzymes to their target substrates and to distinguish the distribution of enzymes in different tumor tissues.
3.7. Electricity Responsiveness
Electricity-sensitive hydrogels can undergo property changes including swelling, deswelling, bending or erosion in response to electric field exposure [24]. Such electricity-responsive hydrogels are usually based on electrically conductive polymers, inorganic conductive nanomaterials and polyelectrolytes [25]. Electrically conductive polymers, such as polyaniline (PANI) and polyacrylamide, are also known as ionic electrosensitive polymers. These polymers can change their redox state upon exposure to electrical signals, causing a change in the charge distribution and conductivity. As a consequence, hydrogels composed of such polymers will undergo an alteration in their shape, volume and drug release. Another strategy used to prepare electricity-sensitive hydrogels is the introduction of conductive inorganic nanomaterials, such as carbon nanotubes, graphene and their derivatives, which not only improve the mechanic properties and electrical conductivity of the hydrogels, but also facilitate the drug release under electric field stimuli.
3.8. Other Responsiveness
Apart from the abovementioned smart hydrogels, other stimulus-responsive in situ hydrogels have been investigated for local regional cancer treatment as well. The in situ hydrogel matrix undergoes physical or chemical changes in response to stimuli such as glutathione [26], and antigen [27], thus leading to swelling, shrinking, deforming or decomposition. The glutathione-responsive hydrogel is a promising platform for localized antitumor therapy because of excessive intracellular glutathione expression. In such platforms, the basic principle is the inclusion of glutathione-reactive groups (e.g., disulfide), which can be cleaved via acceptance of the electrons from the thiol groups in glutathione [28].
In addition, dual and multiple responsive hydrogels for localized antitumor therapy have received more attention than single responsive hydrogels in recent years with the increasing demand for precision therapy. Given the complex tumor microenvironment, these hydrogels are designed to respond to two or more of the abovementioned stimuli for more precise control over the responsive behavior and improvement in the overall performance and applicability of localized drug delivery. Despite these reported multi-stimulus–responsive in situ hydrogels exhibiting excellent antitumor effects and negligible side effects, how to achieve individualized and precise treatment and ensure the reproducibility of the therapeutic effects remain a hard nut to crack. Multi-stimulus–responsive hydrogels are often connected with complicated design and fabrication procedures, which are very common obstacles for novel drug delivery systems being applied in the clinic. Accordingly, how to balance the complex design of in situ smart hydrogels with simple scaled-up industrial production is a very challenging direction for the future.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14102028
References
- Kasinski, A.; Zielinska, P. M.; Oledzka, E.; Sobczak, M. Smart hydrogels - synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541-4572.
- Zhu, J. Q.; Wu, H.; Li, Z.L.; Xu, X.F.; Xing, H.; Wang, M.D.; Jia, H.D.; Liang, L.; Li, C.; Sun, L.Y.; Wang, Y.G.; Shen, F.; Huang, D.; Yang, T. Responsive hydrogel based on triggered click reaction for liver cancer. Adv. Mater. 2022, e2201651.
- Xin, H.; Naficy, S. Drug delivery based on stimuli-responsive injectable hydrogels for breast cancer therapy: A Review. Gels. 2022, 8, 45.
- Li, J.; Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Rossi, S. M.; Murray, T.; McDonough, L.; Kelly, H. Loco-regional drug delivery in oncology: current clinical applications and future translational opportunities. Expert Opin. Drug Deliv. 2021, 18, 607-623.
- Meng, C.; Wei, W.; Wang, Y.; Zhang, K.; Zhang, T.; Tang, Y.; Tang, F. Study of the interaction between self-assembling peptide and mangiferin and in vitro release of mangiferin from in situ hydrogel. Int. J. Nanomed. 2019, 14, 7447-7460.
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460-468.
- Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105-121.
- Patel, P.; Thareja, P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022, 163, 110935.
- Mazidi, Z.; Javanmardi, S.; Naghib, S. M. Mohammadpour Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569.
- Ma, N.; Yan, Z. Research progress of thermosensitive hydrogel in tumor therapeutic. Nanoscale Res. Lett. 2021, 16, 42.
- Xue, B.; Qu, Y.; Shi, K.; Zhou, K.; He, X.; Chu, B.; Qian, Z. Advances in the application of injectable thermosensitive hydrogel systems for cancer therapy. J. Biomed. Nanotechnol. 2020, 16, 1427-1453.
- Mahinroosta, M.; Farsangi, Z. J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42-55.
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Laurenzana, A.; Margheri, F.; Fibbi, G.; Del, R. M.; Bianchini, F.; Calorini, L. The acidic microenvironment as a possible niche of dormant tumor cells. Cell. Mol. Life Sci. 2017, 74, 2761-2771.
- Li, Y.; Song, L.; Lin, J.; Ma, J.; Pan, Z.; Zhang, Y.; Su, G.; Ye, S.; Luo, F.H.; Zhu, X.; Hou, Z. Programmed nanococktail based on pH-responsive function switch for self-synergistic tumor-targeting therapy. ACS Appl. Mater. Interfaces 2017, 9, 39127-39142.
- Li, L.; Scheiger, J. M.; Levkin, P. A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333.
- Andrade, F.; Roca, M. M.; Duran, E. F.; Rafael, D.; Schwartz, S. J. Stimuli-Responsive Hydrogels for Cancer Treatment: The role of pH, light, ionic strength and magnetic field. Cancers. 2021, 13, 1164.
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Del. Rev. 2011, 63, 1257-1266.
- Frachini, E. C. G.; Petri, D. F. Magneto-responsive hydrogels: preparation, characterization, biotechnological and environmental applications. J. Braz. Chem. Soc. 2019, 30, 2010-2028.
- Jahanban, E. R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438-445.
- Gao, F.; Xie, W.; Miao, Y.; Wang, D.; Guo, Z.; Ghosal, A.; Li, Y.; Wei, Y.; Feng, S.S.; Zhao, L.; Fan, H. M. Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv. Healthc. Mater. 2019, 8, 1900203.
- Ferreira, N. N.; Ferreira, L. M.; Cardoso, V. M.; Boni, F. I.; Souza, A. L.; Gremiao, M. P. Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117-133.
- Cao, Z.; Li, W.; Liu, R.; Li, X.; Li, H.; Liu, L.; Chen, Y.; Lv, C.; Liu, Y. pH- and enzyme-triggered drug release as an important process in the design of anti-tumor drug delivery systems. Biomed. Pharmacother. 2019, 118, 109340.
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1-17.
- Carayon, I.; Gaubert, A.; Mousli, Y.; Philippe, B. Electro-responsive hydrogels: macromolecular and supramolecular approaches in the biomedical field. Biomater. Sci. 2020, 8, 5589-5600.
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415-5426.
- Wei, Y.T.; Cui, F.Z.; Tian, W.M. Fabrication and characterization of hyaluronic-acid-based antigen sensitive degradable hydrogel. Front. Mater. Sci. 2009, 3, 353-358.
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. J. Phys-Mater.2019, 2, 032004.
