Obesity associated with a Western diet such as a high-fat diet (HFD) is a known risk factor for inflammatory bowel disease (IBD) and colorectal cancer (CRC). In this study, we aimed to develop fecal microbiome data-based deep learning algorithms for the risk assessment of colorectal dis-eases. The effects of a HFD and a candidate food (Nypa fruticans, NF) on IBD and CRC risk reduction were also evaluated. Fecal microbiome data were obtained from 109 IBD patients, 111 CRC patients, and 395 healthy control (HC) subjects by 16S rDNA amplicon sequencing. IBD and CRC risk as-sessment prediction models were then constructed by deep learning algorithms. Dietary effects were evaluated based on fecal microbiome data from rats fed on a regular chow diet (RCD), HFD, and HFD plus ethanol extracts or water extracts of NF. There were significant differences in taxa when IBD and CRC were compared with HC. The diagnostic performance (area under curve, AUC) of the deep learning algorithm was 0.84 for IBD and 0.80 for CRC prediction. Based on the rat fecal microbiome data, IBD and CRC risks were increased in HFD-fed rats versus RCD-fed rats. Inter-estingly, in the HFD-induced obesity model, the IBD and CRC risk scores were significantly lowered by the administration of ethanol extracts of NF, but not by the administration of water extracts of NF. In conclusion, changes in the fecal microbiome of obesity by Western diet could be important risk factors for the development of IBD and CRC. The risk prediction model developed in this study could be used to evaluate dietary efficacy.
- Microbiology
- Metagenomics
- Dietary efficacy
- Risk assessment
- Fecal microbiome
- Nypa fruticans
- Gut disease
- 16S amplicon sequencing
- obesity
Obesity is an epidemic issue with no country reporting a decrease in prevalence during that period. A Westernized diet that includes a high-sucrose, high-fat diet is known to accelerate obesity-related metabolic syndrome. In previous studies, overweight and obese people have been found to be more susceptible to chronic localized inflammation than those of normal weight.
Inflammatory bowel disease (IBD) is known to be an important risk determinant of developing colorectal cancer (CRC). IBD patients have been observed to have a defective epithelial barrier and increased intestinal permeability. Indeed, studies on human subjects have shown that the gut microbiome is different in patients with IBD from that in healthy control subjects. Metagenomics of gut microbiota in patients with IBD have al-so proven a role of the microbiome in the pathogenesis of IBD.
In this study, the performance of classification for the host phenotype was evaluated by creating a risk assessment model using machine learning techniques based on the stool 16S rRNA metagenomic results of IBD, CRC patients, and healthy controls. In addition, by evaluating the performance of predicting disease risk in a rat model induced by a high-fat diet, an external performance assessment was conducted.
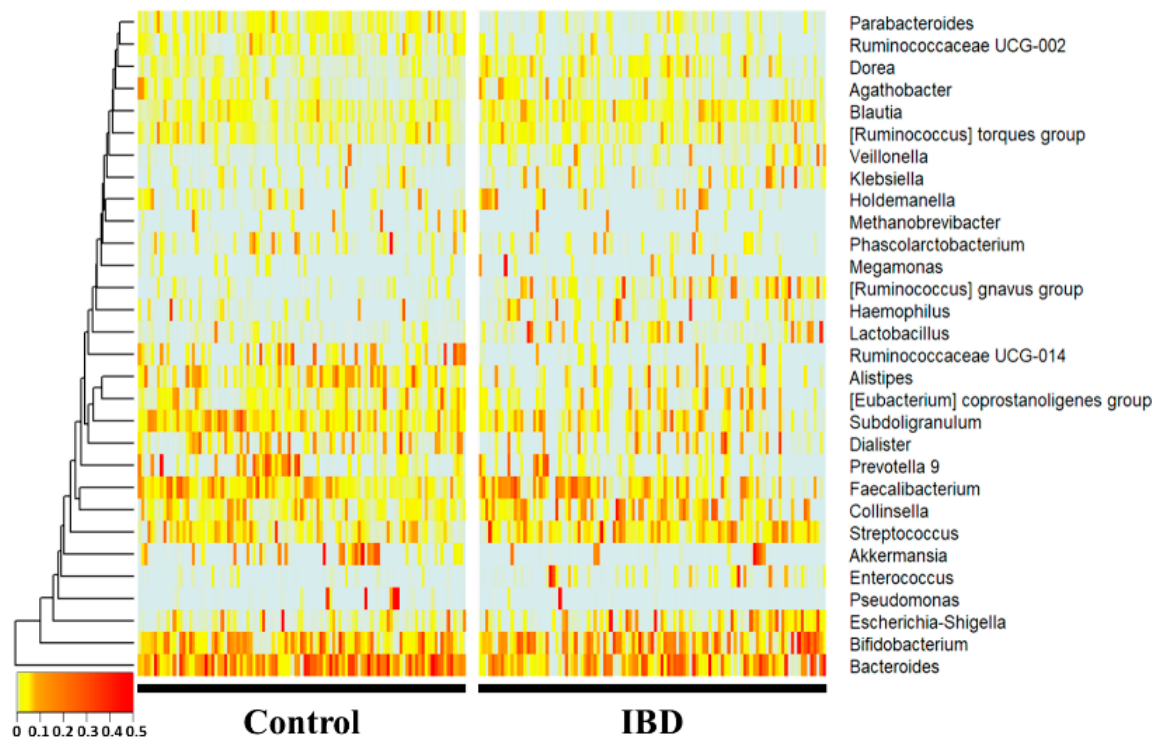
At the genus level, Bacteroides, Subdoligranulum, Alistipes, Ruminococcaceae UCG-014, Parab-acteroides, and Ruminococcaceae UCG-002 were significantly more decreased in the IBD group (p < 0.01), whereas Collinsella, Blautia, [Ruminococcus] gnavus group, Lactobacillus, and Dorea were significantly increased (p < 0.01) compared with the healthy control group. Notably, Enterococcus, [Ruminococcus] gnavus group, Lactobacillus, Ruminococcaceae UCG-014, Parabacteroides, Ruminococcaceae UCG-002, and Alistipes showed drastic fold changes (23-, 13-, 7-, 0.17-, 0.27-, 0.32-, and 0.34-fold, respectively).
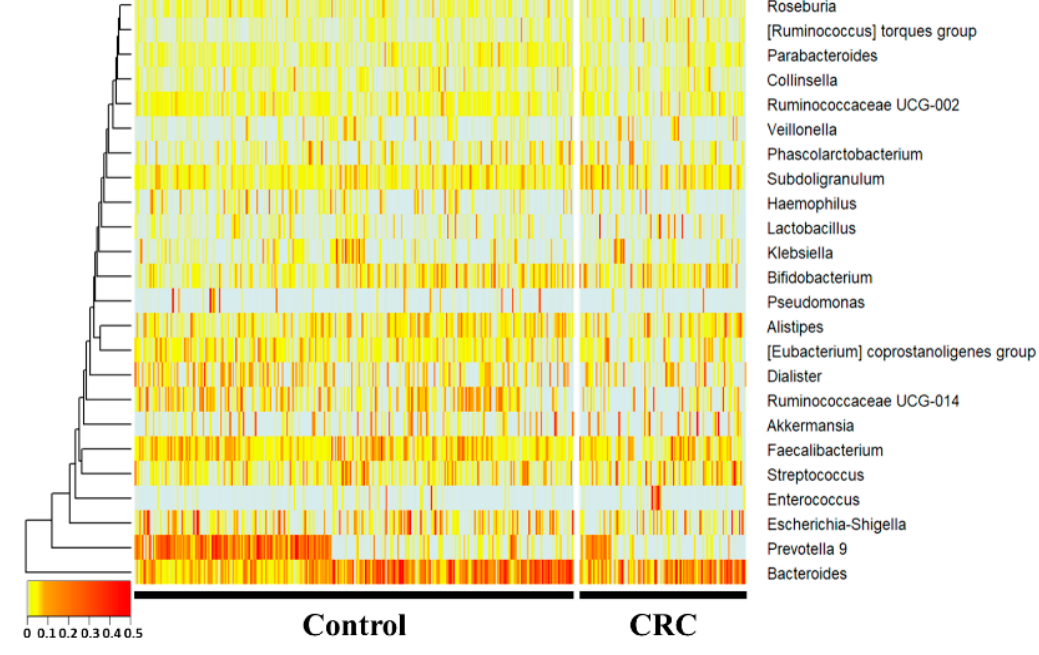
At the genus level, Subdoligranulum and the [Ruminococcus] torques group were increased, whereas Prevotella 9, Ruminococcaceae UCG-014, Haemophilus, and Parabacteroides were sig-nificantly decreased (p < 0.05) in the CRC group compared with the control group. Hae-mophilus, Ruminococcaceae UCG-014, and Parabacteroides showed fold changes of 0.49-, 0.55-, and 0.59-fold, respectively. The [Ruminococcus] torques group, Subdoligranulum, and Prevotella 9 showed 1.7-, 1.4-, and 0.3-fold changes, respectively.
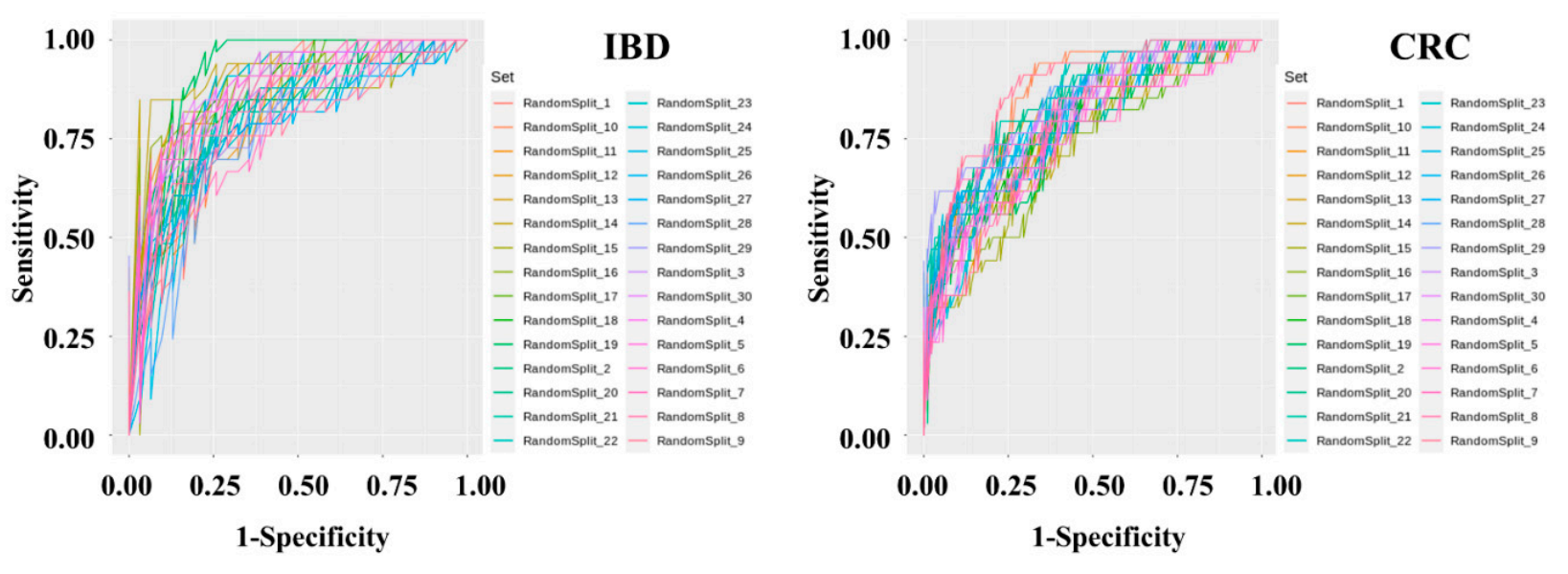
Diagnostic model sets were developed to determine IBD and CRC risk by applying machine learning methods to the stool metagenomic data. The model performance was evaluated based on the resulting receiver operating characteristic (ROC) curve. The AUC values from 30 iterations of the test were plotted as a box plot . The disease classification performance of the IBD risk model was slightly better than that of the CRC risk model. In the IBD model, the mean AUC, standard deviation, minimum, and maximum of the model were 0.836, 0.0437, 0.7429, and 0.9277, respectively. In the CRC model, the mean AUC, standard deviation, minimum, and maximum of the model were 0.7982, 0.0359, 0.7757, and 0.9462, respectively. The specificity and sensitivity of the set showing the highest AUC in the IBD model were 0.7419 and 0.9091, respectively. The specificity and sensitivity of the set showing the highest AUC in the CRC model were 0.8409 and 0.7059, respectively.
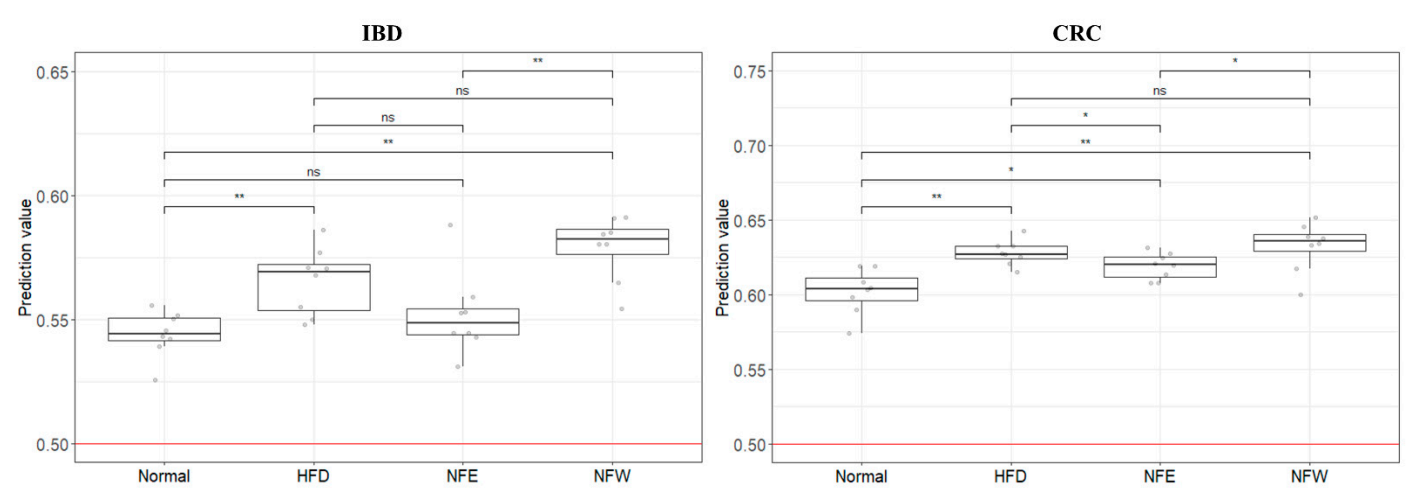
To determine whether the colorectal disease risk assessment model could evaluate well in a rigorous animal model, we calculated predictive values by substituting the rat stool metagenome data from the normal diet, HFD, NFE, and NFW groups. In both models for evaluating IBD and CRC risk, the predicted values in all groups were higher than 0.5, the standard for distinguishing the healthy control group from the patient group. However, the predicted risk score was increased in the HFD group compared with the normal diet group. Among the groups taking NF with HFD, the NFW group tended to show increased risk scores while the NFE group tended to show a level lower than or similar to the normal group. This trend was more conspicuously observed in the IBD model than in the CRC model.
In the present study, we constructed a deep learning-based risk prediction model using fecal microbiome data from IBD and CRC patients. The AUC value used as predictive performance of the deep learning algorithm of the model was 0.84 for IBD and 0.80 for CRC. Although the microbial profiles from normal individuals and patients with IBD are sometimes obtained and compared, this comparison is seldom performed to examine the performances of these prediction models in controlled animal models. We further evaluated the risk by applying this predictive model for colonic disease to a controlled obese rat model. The risk prediction model for IBD and CRC showed that the HFD group increased the risk compared to the regular diet group in the rat experiment. This is an important finding because it suggests that microbiome compositions induced by a high-fat diet and those of IBD and CRC are shared. Additionally, we assessed whether the consumption of NF extract reduced the risk of CRC or IBD when consumed during a high-fat diet. Our results confirmed the correlation that the intake of ethanol-extracted NFs could increase the microbiome composition, which lowers the IBD risk score. The novel approach suggests that disease risk prediction models can be established using well-controlled animal models and used as tools to assess dietary efficacy through changes in microbiome composition.
Our study has some limitations. A risk assessment model was created based on a human-derived fecal microbiome profile. However, its risk assessment was applied to a rat animal model. In addition, controlled clinical samples are extremely rare, making it an inevitable choice. Furthermore, although there are various models of colitis (UC/CD) in murine, these methods are mainly postulated to chemically alter the microbial profile by disrupting the mucosal barrier and inducing inflammation of the colon via intestinal permeation by symbiotic microbiota . On the other hand, the HFD rat model, which can induce chronic inflammation by natural intestinal flora, was selected as a controlled external validation model for our colorectal cancer and colitis risk assessment model. When the four groups were scored with the IBD and CRC risk assessment models based on identical fecal microbiome composition data from obese rats, the IBD model distinguished each group better than the CRC model. This leads us to infer that the microbiome induced by the HFD model is relatively correlated with IBD. However, in the case of CRC, the obesity model appears to be an unsuitable model for risk assessment. It is judged that the level of evaluation is not dramatic because cancer develops through a long process. If possible, controlled clinical samples would be more appropriate for risk assessment.
In conclusion, this study demonstrates that the risk prediction model generated based on the microbial profile of clinical disease can be externally validated by utilizing appropriate animal disease models. This study proves that a well-assessed microbiome profile-based prediction model can be a platform for rapidly estimating various indicators or markers or the efficacy of diets and foods.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091833
