The restoration of an intact epidermal barrier after wound injury is the culmination of a highly complex and exquisitely regulated physiological process involving multiple cells and tissues, overlapping dynamic events and protein synthesis and regulation. Central to this process is the cytoskeleton, a system of intracellular proteins that are instrumental in regulating important processes involved in wound repair including chemotaxis, cytokinesis, proliferation, migration, and phagocytosis. One highly conserved family of cytoskeletal proteins that are emerging as major regulators of actin and microtubule nucleation, polymerization, and stabilization are the formins. The formin family includes 15 different proteins categorized into seven subfamilies based on three formin homology domains (FH1, FH2, and FH3). The formins themselves are regulated in different ways including autoinhibition, activation, and localization by a range of proteins, including Rho GTPases
1. Formins in Inflammation
Formins are involved in the regulation of the inflammation phase of healing through their fundamental role in controlling cell polarity, dynamics, and the migration of inflammatory cells (
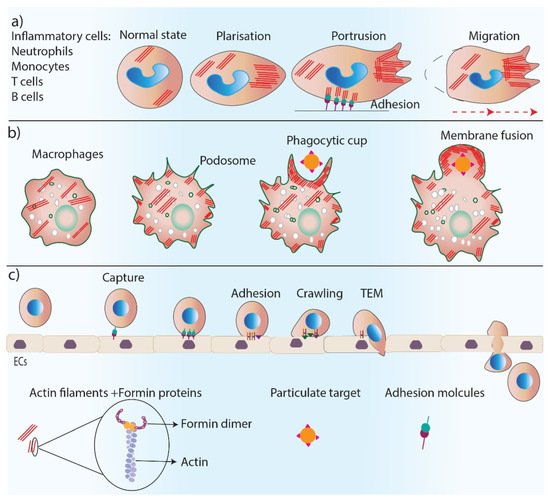
Figure 3). Neutrophils are the first inflammatory cells that migrate towards the wound bed from blood vessels, and actin reorganization plays an essential role in neutrophil chemotaxis [
42]. The predominant actin nucleating proteins found in neutrophils are Dia proteins [
43,
44]. The deletion of mDia1 has been shown to impair neutrophil polarization and directed migration, a function that was found to be associated with WASP at the leading edge of these cells [
43]. Leukocytes such as neutrophils, B and T cells, and monocytes migrate to the wound site from blood vessels, a process called transendothelial migration (TEM) [
45]. TEM is a multi-step process that includes the capture of leukocytes on endothelial cells by rolling, crawling, and the adhesion of leukocytes on an endothelial monolayer and eventually migrating over the monolayer [
45]. The depletion of mDia1 impairs the ability of cells to undergo TEM [
46].
Figure 3. Formins’ role in inflammation. Formins play an essential role in inflammation by regulating actin polymerization in inflammatory cells. (a) Formins control cell polarity, cell protrusions and directed migration in inflammatory cells. (b) Formins are involved in podosome and phagocytic cup formation and the phagocytosis process. (c) Trans-endothelial migration of inflammatory cells requires several steps including, cell capture, adhesion, crawling and TEM which are all regulated by formin-mediated actin polymerization.
Macrophages are additional important inflammatory cells that are recruited to the wound to remove pathogens and debris through their phagocytosis ability [
47]. Macrophages have actin-rich protrusions, called podosomes, which are adhesion structures that facilitate tissue invasion and macrophage movements through complex tissues for immune surveillance [
48]. The phagocytic uptake of antigens by macrophages depends on the polymerization of actin filaments [
49]. Formins (FMNL1) are the main regulator of actin reorganization in podosomes, and any reduction in FMNL1 activity disrupts podosome structures [
50]. Macrophage phagocytosis also relies on the activity of formins (mDia1, mDia2, FMNL1) [
51,
52], which are enriched at macrophage pseudopodia and regulate actin re-organization in the phagocytic cup during complement receptor (CR3)-mediated phagocytosis as a downstream effector of RhoA–ROCK signaling [
53,
54].
The migration and entry of lymphocytes, including B and T cells, to damaged tissue, is essential for the adaptive immune response of the body. Impaired T cell trafficking is observed in FMNL1 knock-out mouse models, which were shown to have inflamed tissues, indicating the important role of formins in T-cell morphology and mobility. This role is likely due to formin’s function in actin nucleation and polymerization at the back of migrating T cells [
55]. Indeed, T cells of mDia1−/− mice have reduced actin polymerization in vitro, and T cell trafficking is disrupted and inefficient in vivo [
56]. Diminished T cell populations in lymphoid tissues have also been observed in DRF1−/− mice. Isolated T cells from the spleen of DRF1−/− mice were less adhesive to the extracellular matrix and showed impaired migration [
57]. mDia1−/− mice also have impaired adhesion and spread to the cellular matrix in dendritic cells. Furthermore, T-cell stimulation is also impaired in these mice [
58].
Formins are important regulators of the T cell synapse. Actin assembly and cytoskeleton rearrangement are involved in immunological synapses [
59]. mDia1 and FMNL1 have been found to be localized in the lamellipodium of T cells, forming the immunological synapse [
60]. These formins also regulate MTOC polarization in T cells when they encounter an antigen-presenting cell (APC) in immunological synapses [
60].
2. Formins in Skin Cell Migration
Cell migration relies on the reorganization of the actin cytoskeleton into complex actin-rich structures, such as filopodia and lamellipodia, at the front edge of migrating cells [
3]. These thin protrusive extensions are required for directed migration, exploring the extracellular matrix, and penetrating tissue spaces. They are also well suited for intercalating between cells, such as during the migration of leukocytes across endothelial layers [
64]. Formins are involved in filopodia formation, which is a highly dynamic process creating thin protrusions that are rich in parallel unbranched actin filaments [
65]. The extension of these protrusions occurs by the elongation and capping of the barbed ends of actin filaments [
66]. Rho GTPase family proteins are known to be the main regulator of filopodia formation and rearrangements [
67]. Rho GTPase blocks the autoinhibitory switch of DRFs, and active DRFs are able to nucleate actin filaments [
29] and cap the barbed ends by their FH2 domain, stabilizing the formation of an adjacent actin dimer [
68]. The capping action allows the actin nucleus to elongate from its barbed end [
29,
69]. The role of formins in filopodia formation can be disrupted by the interaction between formins and either the WASP or Arp2/3 complex, which strikes a balance between the formation of filopodia and lamellipodia in migrating cells [
67,
70].
Lamellipodia are actin-rich protrusions that are composed of a branched actin filament meshwork assembled by the Arp2/3 complex and the WASP family [
71]. However, several studies show an important role for formins (mainly mDia2) in the formation of lamellipodia, which is mediated by nucleating the actin filaments and protecting them from capping [
71,
72].
In addition to actin filaments, formins (mainly mDia1 and mDia2) regulate microtubule dynamics, which is essential for cell polarity and directed migration. Not only do formins bind and stabilize the microtubules [
73], but mDia1 has been found to polarize microtubules from the cell center microtubule-organizing center (MTOC) to the periphery in migrating cells towards the direction of cell migration [
74,
75]. Furthermore, mDia2 stabilizes microtubules, which is essential for cell migration to occur [
34].
3. Formins in Cell Proliferation
To replace damaged tissue during wound healing, cell proliferation occurs, leading to the restoration of the epidermis and the formation of new dermis through the production of granulation tissue [
81]. Cytokinesis is the final stage of cell division that divides the cytoplasm of a cell into two cells following mitosis. Cytokinesis begins with the assembly of an actomyosin-rich contractile ring. When the ring contracts, a cleavage furrow forms, which eventually separates the two sides of the ring [
82]. The positioning and induction of a cleavage furrow on the metaphase plate are regulated by microtubules [
83]. Clusters of recycled endosomes are required as the main source of the additional membrane around the cleavage furrow to increase cell surface area and the accommodation of the cell shape and polarity changes during cytokinesis [
84]. The accumulation of endosomes occurs in the midbody area near MTOC, which relies upon activity of microtubule motors [
85].
Formins play a critical role in cytokinesis. Several studies have shown the failure of cytokinesis following a mutation or the genetic deletion of formin proteins. There are different functions of formin proteins during cytokinesis, which rely on their role in actin reorganization and microtubule stabilization. Rho-regulated DRFs are the main stimulator of actin assembly in the contractile cortex [
86]. Formins have been identified as essential factors for the formation/activity of contractile rings during cytokinesis in drosophila [
87,
88], C. elegans [
89], yeasts [
90], and mammals [
91]. DRFs localize in pericentrosomal dividing cells near the contractile ring and furrow, which is mediated by Rho-GTPase regulation [
92]. mDia1 and mDia2 localize to the microtubules of proliferating cells and facilitate cytokinesis by stabilizing the microtubules [
93]. Formins are also known to act as a link between microtubules and actin filaments during cytokinesis and regulate their positioning. The overexpression of formins leads to the disruption of the alignment of microtubules and actin filaments [
94]. A study on fibroblasts and Xenopus embryos showed that the Rhod–hDia2C–Src pathway involves the interaction of endosomal vesicles with microtubules and actin [
95]. In addition, RhoA-activated DRFs are involved in the stability of the cytokinesis furrow by assembling β-actin filaments at the site of cytokinesis and directly at the furrow [
96].
4. Formins in Epithelial-to-Mesenchymal Transition (EMT)
Epithelial-to-mesenchymal transition (EMT) is a vital part of the wound-healing process that occurs during re-epithelization and is mediated by inflammatory cells and fibroblasts [
97]. During re-epithelization, keratinocytes proliferate and migrate to restore the epithelial barrier. Re-epithelialization is supported by the conversion of cells from a stationary state to a migratory one, mediated by EMT [
98]. Keratinocytes go through cytoskeleton rearrangement, lose their polarity and cell–cell adhesions, modulate their interaction with the ECM, and obtain mesenchymal features [
99]. The cytoskeleton rearrangement during EMT is regulated by transforming growth factor β1 (TGF-β1) and its downstream effectors RhoA GTPase and formins including DIAPH1 and DIAPH3 FHOD1 and FMNL2 [
100,
101,
102].
5. Formins in Angiogenesis
The process of new blood vessel formation, called angiogenesis, is critical for effective wound healing. Following the resolution of inflammation, newly branched blood vessels invade granulation tissue to provide nutrition and oxygen to the newly formed tissue [
103]. Actin reorganization is required for endothelial cell (EC) polarization, proliferation, migration, and adherence [
3]. Formins are one of the cytoskeletal regulators of angiogenesis with conflicting effects on this process. Due to formin’s role in actin polymerization, these proteins, especially FMNL3, are required for filopodia formation in migrating endothelial cells during angiogenesis [
104]. Several studies show an extending role of formins beyond their effect on EC migration. ECs are highly flattened cells in a quiescent state. However, during angiogenesis, these cells undergo a change in their morphology and polarity in order to initiate migration [
105]. EC microtubules realign and stabilize during angiogenesis, leading to a change in the morphology of ECs. Formin screening has identified FMNL3 as the regulator of angiogenic ECs′ morphogenesis, and silencing FMNL3 has led to the inhibition of blood vessel formation. FMNL3 has been shown to act as a downstream effector of Cdc42 and RhoJ and regulates microtubule alignment during EC morphogenesis [
106,
107]. During angiogenesis, ECs establish cell–cell junctions and rearrange for new vessel formation and stabilization. Actin filaments polymerize and assemble in these EC junctions. FMNL3 has been shown to localize in EC junctions where they promote actin filament polymerization. FMNL3 knockdown has also led to impaired actin filaments′ polymerization and stabilization highlighting the importance of this formin in maintaining EC junctions [
108].
DAAM1 formin has been identified as a promoting factor of both the microtubule stabilization and actin polymerization of ECs. However, the overexpression of DAAM1 elevates microtubule stabilization rather than actin polymerization and inhibits angiogenesis by inhibiting the proliferation and migration of ECs [
109].
Growth factors such as vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) promote angiogenesis through two different signaling pathways. VEGF increases endothelial cell permeability through the activation of Src kinase via its receptor VEGFR. Ang-1 inhibits VEGF-stimulated permeability by activating RhoA and its downstream effector mDia [
110,
111]. mDia has been shown to interact directly with Src and inhibits its activity by sequestering it from the VEGFR pool, which ultimately blocks EC permeability and promotes barrier integrity. This function of Ang-1 is important in protecting blood vessels in chronic wounds with persistent inflammation [
111].
6. Formins in Tissue Maturation and Fibrosis
Maturation is the final stage of wound healing, which involves the remodeling of granulation tissue. Myofibroblast differentiation is a crucial aspect of this process as they promote wound contraction and the realignment of ECM components, which restores tissue integrity. Fibroblasts differentiate into myofibroblasts under the mechanical stress of the ECM. During this transition, myofibroblasts develop highly organized and contractile actin filament bundles called stress fibers [
112]. Stress fibers are assembled by formin (mDia1/mDia2)-driven actin polymerization at focal adhesions, and silencing mDia1/2 in stress fibers disrupts myofibroblast differentiation [
113]. One of the main regulators of myofibroblast differentiation is TGF-β1, which promotes the formation of stress fibers and giant focal adhesions in myofibroblasts [
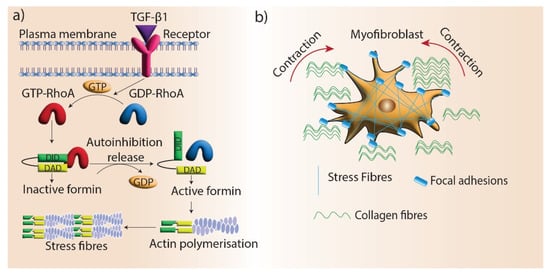
114]. mDia proteins are recruited to stress fibers via TGF-β1/RhoA signaling and facilitate myofibroblast differentiation by promoting actin filament polymerization (
Figure 4). In addition, myofibroblast differentiation is regulated by the interactions of the microtubule system and actin cytoskeleton via mechanical coupling. TGF-β1 signaling is blocked by microtubule polymerization, preventing myofibroblast differentiation. mDia2 interacts with microtubules and its localization to stress fibers can be regulated by microtubules [
115].
Figure 4. Formins’ role in myofibroblast contraction. (a) Stress fiber generation by formin-mediated actin assembly. TGF-β1 signaling promotes stress fiber formation in myofibroblasts by mediating the activation of RhoGTPase, which blocks the autoinhibition of formins and promotes actin assembly. (b) Stress fibers locate in the focal adhesions of myofibroblasts and facilitate the contraction and maturation of new ECM.
This entry is adapted from the peer-reviewed paper 10.3390/cells11182779