Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Many algae and invertebrate species have long been used as human food, animal fodder and sources of valuable substances, including lipids. Marine seaweeds and invertebrates are rich in unusual lipids, steroids, triterpenoids, phospholipids, glycolipids, and polyunsaturated FA and are of potential value as sources of essential FA, important in the nutrition of humans and animals. In addition, proteins of marine algae and invertebrates, which are natural reservoirs of bioactive peptides, are of great interest.
- bacteria
- microalgae
- fungal endophytes
- lipopeptides
1. Fatty Acids Derived from Seaweed Lipopeptides
Marine and freshwater algae are a phylogenetically heterogeneous group of aquatic plants that belong to three main taxonomic groups: green (Chlorophyta), brown (Phaeophyta), and red (Rhodophyta) [226]. Since ancient times, seaweeds have been of great practical interest since they contain bioactive elements such as iodine, bromine or chlorine, and metabolites, steroids, carotenoids, fatty acids, lipopeptides, alkaloids, and other organic molecules that have antimicrobial, antiviral, anti-inflammatory and immunotropic properties [227,228,229,230,231].
Two cyclic lipopeptides, mebamamide A and B, containing FA (108, for structure see Figure 26, and activity see in Table 11) and (3R,8S)-3,8-dihydroxy-9-methyldecanoic acid (109), respectively, were isolated from the green alga Derbesia marina [232].
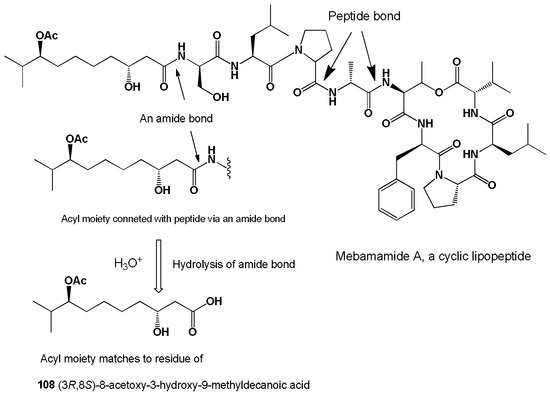
Figure 26. Graphical display of the chemical structure of the green alga Derbesia marina lipopeptide and the free FA formed by hydrolysis of the amide bond.
Table 11. Predicted biological activity of FA from peptides of seaweeds and molluscs.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 108 | Hypolipemic (0.858); Lipid metabolism regulator (0.835); Anti-hypercholesterolemic (0.634) Antifungal (0.648); Atherosclerosis treatment (0.603) |
| 109 | Hypolipemic (0.858); Lipid metabolism regulator (0.835); Anti-hypercholesterolemic (0.634) Antifungal (0.648); Atherosclerosis treatment (0.603) |
| 110 | Preneoplastic conditions treatment (0.779); Hypolipemic (0.775); Anesthetic general (0.772) Lipid metabolism regulator (0.768); Acute neurologic disorders treatment (0.694) |
| 111 | Preneoplastic conditions treatment (0.805); Acute neurologic disorders treatment (0.723) Antiviral (Arbovirus) (0.716); Anti-inflammatory (0.650); Antiviral (Picornavirus) (0.649) |
| 112 | Lipid metabolism regulator (0.890); Hypolipemic (0.870); Anti-hypercholesterolemic (0.802) Atherosclerosis treatment (0.692); Cholesterol synthesis inhibitor (0.511) |
| 113 | Lipid metabolism regulator (0.895); Preneoplastic conditions treatment (0.778) Anti-hypercholesterolemic (0.777); Hypolipemic (0.758); Atherosclerosis treatment (0.683) |
| 114 | Mucositis treatment (0.886); Anesthetic general (0.852); Lipid metabolism regulator (0.842) Autoimmune disorders treatment (0.798); Transplant rejection treatment (0.795) |
| 115 | Anti-ischemic, cerebral (0.943); Acute neurologic disorders treatment (0.797) Anticonvulsant (0.702); Anti-hypercholesterolemic (0.642); Antihypertensive (0.627) |
| 116 | Lipid metabolism regulator (0.822); Vasodilator, peripheral (0.803); Vasoprotector (0.793) Hypolipemic (0.757); Anti-hypercholesterolemic (0.677); Atherosclerosis treatment (0.647) |
| 117 | Lipid metabolism regulator (0.890); Hypolipemic (0.870); Anti-hypercholesterolemic (0.802) Atherosclerosis treatment (0.692); Cholesterol synthesis inhibitor (0.511) |
* Only activities with Pa > 0.5 are shown.
The sacoglossan mollusc Elysia rufescens is known to use the green algae Bryopsis pennata and B. plumosa as its main diet [233,234,235,236,237]. Analysis of lipid extracts from molluscs and algae showed that they contain biologically active cyclic depsipeptides, (kahalalides A–F, iso-KF, 5-OHKF, K, O–S, R′, S′, W, and Y) and five linear depsipeptides (kahalalides G, H, J, V, and X), which exhibit cytotoxic, antitumour, antimicrobial, antileishmanial and immunosuppressive activities [233].
The (R)-2-methylbutanoic acid (110, for structures see Figure 27, and biological activity is shown in Table 11) was found in kahalalide A and 5-methylhexanoic acid (111) was included in the structure of the kahalalides B, F, G, O, R2 and S2. 3-Hydroxy-9-methyldecanoic acid (112) was present in kahalalides E, H, J, K, and Y. (R)-4-Methylhexanoic acid (113), 5-hydroxy-5-methylhexanoic acid (114), (S)-2-hydroxy-9-methyldecanoic acid (115), 5-hydroxy-7-methyloctanoic acid (116), and (R)-3-hydroxy-7-methyloctanoic acid (117) were incorporated into the lipopeptides iso-kahalalide F, 5-OH-kahalalide F, kahalalide P and Q, kahalalide R1 and S1, and kahalalide V, respectively. As specimens, Figure 28 shows the green alga Bryopsis pennata, B. plumosa and the sacoglossan mollusc Elysia rufescens.
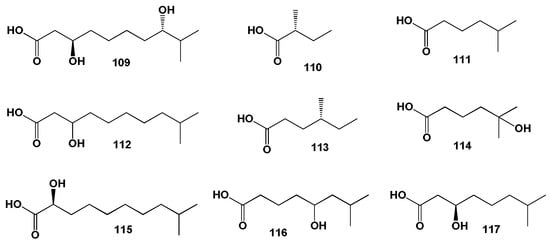
Figure 27. FA incorporated into lipopeptides derived from lipid extracts of the green algae Bryopsis pennata, B. plumosa and the sacoglossan mollusc Elysia rufescens.

Figure 28. The green algae Bryopsis plumosa (a) and B. pennata (b) are the staple food for the sea slug, Elysia rufescens (c,d). This mollusk is similar to nudibranch, but is not classified in this order of gastropods, but belongs instead to a closely related clade, Sacoglossa. These molluscs synthesize a class of cyclic depsipeptides called kahalalides.
According to the PASS data, FA (115) showed properties as a cerebral anti-ischemic agent with a confidence level of more than 94%. This FA is found in cyclic depsipeptides, kahalalides P and Q, and a 3D graph of its predicted and calculated cerebral anti-ischemic activity is shown in Figure 29.

Figure 29. 3D Graph showing the predicted and calculated cerebral anti-ischemic activity of FA (115). This acid is a fragment of cyclic depsipeptides, kahalalides P and Q.
2. Fatty Acids Incorporated into Lipopeptides of Marine Sponges
Marine and freshwater sponges (class Demospongiae) are known to be home to many symbiotic microorganisms, including fungal endophytes, bacteria, and some unicellular organisms. Sponges, including their symbiotic microorganisms, synthesize many secondary metabolites such as steroids, terpenoids, carotenoids, halogenated and unusual fatty acids, alkaloids, and of course cyclic and linear lipopeptides [1,4,6,17,23,58,60,61,65,66,128,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215].
The marine sponges of the genus Theonella synthesize a wide variety of lipopeptides, and in the sponge Theonella aff. mirabilis, a pentapeptide was found that contained a rare (2R,3R)-aziridine-2,3-dicarboxylic acid (118, see Figure 30) [238]. The isolated lipopeptide inhibits the proteolytic activity of trypsin-like serine proteases, papain-like cysteine proteases, and pepsin-like aspartyl proteases [239]. Previously, this FA (118) was found and isolated from the ascomycete Streptomyces sp. MD 398-A1 [240]. A similar lipopeptide was isolated from the Red Sea sponge Theonella swinhoei (order Lithistida, see Figure 31) and is a potent inhibitor of cathepsin B, protease, and HIV [241]. The aziridine-containing compounds are powerful immuno-modulatory and anticancer agents and are of practical interest to pharmacologists [242,243].
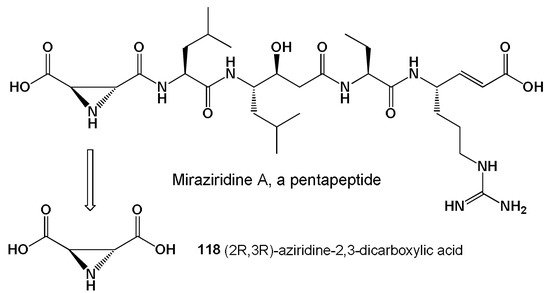
Figure 30. Graphical display of the chemical structure of lipopeptide isolated from the marine sponge Theonella aff. mirabilis and the free FA formed by hydrolysis of the amide bond.

Figure 31. Samples of marine sponges: (a), Theonella cylindrica; (b), T. swinhoei; (c), T. swinhoei; (d), T. swinhoei. It is known that sea sponges from the genus Theonella are home to many associated bacteria that occupy up to 40% of their body volume. Entotheonella sp. (Tectomicrobia) is a filamentous symbiont that produces almost all known biologically active compounds derived from the sponge Theonella swinhoei.
2.1. Saturated, Methyl-Branched, and Unsaturated Fatty Acids
We have previously mentioned that neo fatty (carboxylic) acids have been isolated from cyanobacteria, microalgae, and some marine invertebrates [128]. Highly cytotoxic polypeptides named polytheonamides A and B are found in extracts of the marine sponge Theonella swinhoei [244,245,246,247]. Both polypeptides are quite unusual in that one peptide molecule contains nine amino acids with tert-butyl units, and both linear polypeptides contain a rare neo-FA, 5,5-dimethyl-2-oxohexanoic acid (119, for structure see Figure 32, and activity see in Table 12).
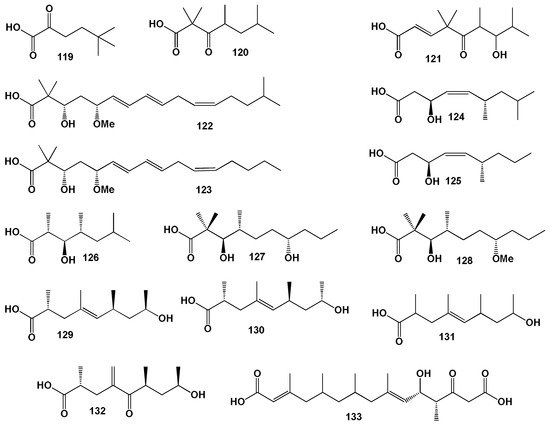
Figure 32. Branched, saturated, neo-, and unsaturated FA isolated from sponge lipopeptides.
Table 12. Predicted biological activity of FA from peptides of marine sponges.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 118 | Antineoplastic (0.883); Lipid metabolism regulator (0.836); Anti-inflammatory (0.845) Apoptosis agonist (0.847); Acute neurologic disorders treatment (0.795); Antifungal (0.793) |
| 119 | Phobic disorders treatment (0.859); Psychostimulant (0.731); Antiviral (0.731) Acute neurologic disorders treatment (0.586); Neuroprotector (0.574) |
| 120 | Antiarthritic (0.805); Preneoplastic conditions treatment (0.730); Sclerosant (0.726) Acute neurologic disorders treatment (0.696); Anti-inflammatory (0.641) |
| 121 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) Cytoprotectant (0.668); Antiviral (Picornavirus) (0.585); Hypolipemic (0.575) |
| 122 | Lipid metabolism regulator (0.880); Antineoplastic (0.863); Hypolipemic (0.816) Anti-hypercholesterolemic (0.672); Atherosclerosis treatment (0.590) |
| 123 | Lipid metabolism regulator (0.924); Antineoplastic (0.873); Hypolipemic (0.839) Anti-hypercholesterolemic (0.642); Atherosclerosis treatment (0.592) |
| 124 | Hypolipemic (0.911); Lipid metabolism regulator (0.829); Anti-inflammatory (0.765) Anti-hypercholesterolemic (0.718); Acute neurologic disorders treatment (0.715) |
| 125 | Lipid metabolism regulator (0.929); Hypolipemic (0.908) Anti-hypercholesterolemic (0.825); Atherosclerosis treatment (0.680) |
| 126 | Antineoplastic (0.788); Hypolipemic (0.754); Acute neurologic disorders treatment (0.687) |
| 127 | Dermatologic (0.909); Anti-psoriatic (0.888); Anti-eczematic (0.856); Antifungal (0.605) |
| 128 | Anti-psoriatic (0.862); Antineoplastic (0.846); Antifungal (0.625); Anti-eczematic (0.601) |
| 129 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antiviral (Arbovirus) (0.654) |
| 130 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antibacterial (0.555) |
| 131 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antibacterial (0.555) |
| 132 | Antifungal (0.688); Antiprotozoal (Plasmodium) (0.570); Antibacterial (0.514) |
| 133 | Antineoplastic (0.876); Antifungal (0.771); Lipid metabolism regulator (0.763); Hypolipemic (0.707) |
* Only activities with Pa > 0.5 are shown.
Linear peptides named yakuamides A and B were found and isolated from extracts of the Japanese sponge Ceratopsia sp. and they showed activity against P388 murine leukemia cells [245] and both contained 2,2,4,6-tetramethyl-3-oxoheptanoic acid (120).
The marine sponge Poecillastra sp. (Bahamas) yielded the potently cytotoxic poecillastrins A–C, which are related to the chondropsin D, and the closely related cytotoxic poecillastrin D was isolated from Jaspis serpentina (Oshimashinsone, Japan) [248]. All the above mentioned lipopeptides contain (E)-7-hydroxy-4,4,6,8-tetramethyl-5-oxonon-2-enoic acid (121).
Rare cyclic lipodepsipeptides, lipodiscamides A and C, were found in extracts of the marine sponge Discodermia kiiensis. Lipodiscamides A and C contain (3S,5R,6E,8E,11Z)-3-hydroxy-5-methoxy-2,2,15-trimethylhexadeca-6,8,11-trienoic acid (122), and lipodiscamide B contains FA (123) [249].
Two “head-to-side-chain” depsiundecapeptides named stellatolide A and B were present in lipid extracts from the marine sponge Ecionemia acervus. Both compounds showed strong antiproliferative activity against three human cancer cell lines (Lung-NSCLC A549, Colon HT-29 and Breast MDA-MB-231). (3S,6S,Z)-3-Hydroxy-6,8-dimethylnon-4-enoic acid (124) was isolated from stellatolide A, and (3S,6S,Z)-3-hydroxy-6-methylnon-4- enoic acid (125) was found in stellatolide B [250].
HIV-inhibitory cyclic depsipeptides known as neamphamide A–C were isolated from Papua New Guinea in the marine sponge Neamphius huxleyi. All lipopeptides contain 2R,3R,4R)-3-hydroxy-2,4,6-trimethylheptanoic acid (126) [251].
Cyclic depsipeptides named halipeptin A and B were found in extracts from the marine sponge Haliclona sp. (see Figure 33). (3R,4R,7S)-3,7-Dihydroxy-2,2,4-trimethyldecanoic acid (127) was present in halipeptin B and C, and (3R,4R,7S)-3-hydroxy-7-methoxy-2,2,4-trimethyldecanoic acid (128) was found in halipeptin A and D [252].

Figure 33. The marine sponges belonging to the genus Haliclona contain more than fifty species of actinobacteria belonging to the genera Streptomyces, Nocardiopsis, Micromonospora and Verrucosispora. Members of this genus produce large amounts of bioactive metabolites such as lipids, steroids, FA, lipopeptides and amino acids.
Cytotoxic depsipeptides, seragamides A–F, containing (2R,6S,8R,E)-8-Hydroxy-2,4,6-trimethylnon-4-enoic acid (129) were detected in the lipid extracts in the Okinawan sponge Suberites japonicus. The same FA has been found in jasplakinolide D, M, Q, and R1, as well as in cyclic depsipeptides named geodiamolides J, P, and R, which have been isolated from the marine sponge Cymbastela sp. and found in geodiamolides A and B from the sponge Geodia sp., and geodiamolide D from the sponge Pseudoaxinyssa sp. [253].
A cyclic depsipeptide, Jaspamide (jasplakinolide), containing (2R,6S,8S,E)-8-hydroxy-2,4,6-trimethylnon-4-enoic acid (130) was found in the lipid fraction of Fijian sponges of the genus Jaspis [254], and a similar peptide was found in other types of sponges [255]. The cytotoxic peptides, jaspamide and geodiamolide TA containing (E)-8-hydroxy-2,4,6-trimethylnon-4-enoic acid (131), found in the lipid fraction of the sponge Hemiasterella, while lipopeptides the geodiamolides J, K, and jaspamide B containing (2R,6S,8R)-8-hydroxy-2,6-dimethyl-4-methylene-5-oxononanoic acid (132) were isolated as minor metabolites from the sponge Cymbastela sp. [256].
Homophymines A–E and A1–E1 are a series of cyclodepsipeptides isolated from Homophymia sp. collected from shallow waters off the east coast of New Caledonia [257,258]. They are similar in structure to the previously published antiviral marine cyclodepsipeptides callipeltin A, neamphamide A, papuamides, theopapuamides, and mirabamides [259,260,261,262,263,264,265]. Homophymine A was cytotoxic against uninfected PBMC cells with an IC50 of 1.19 μM, but it was almost sixteen times more effective against infected cells and exhibited potent cytotoxicity with IC50 values ranging from 2 to 100 nM. These compounds were the most potent against the PC3 human prostate adenocarcinoma and the SK-OV3 human ovarian adenocarcinoma cell lines [257,258,259,260,261,262,263,264,265]. (2S,3S,4S,6S)-3-Hydroxy-2,4,6-trimethyloctanoic acid (134, for structure see Figure 34, and activity is shown in Table 13) was isolated from the homophymines A and A1, (2S,3S,4S)-3-hydroxy-2,4,6-trimethylheptanoic acid (135) from the homophymines B and B1, (2S,3S,4S,6S)-3-hydroxy-2,4,6-trimethylnonanoic acid (136) from the homophymines C and C1, (2S,3S,4S,6S)-3-hydroxy-2,4,6,8-tetramethylnonanoic acid (137) from the homophymines D and D1, and (2S,3S,4S,6S,8S)-3-hydroxy-2,4,6,8-tetramethyldecanoic acid (138) was isolated from the homophymines E and E1.
Figure 34. Branched, unsaturated and glycosidic FA derived from sponge lipopeptides.
Table 13. Predicted biological activity of FA derived from sponge peptides.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 134 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 135 | Sclerosant (0.835); Antineoplastic (0.788); Hypolipemic (0.754); Anti-inflammatory (0.716) |
| 136 | Sclerosant (0.853); Hypolipemic (0.824); Antineoplastic (0.775); Anti-inflammatory (0.734) |
| 137 | Sclerosant (0.815); Hypolipemic (0.807); Antineoplastic (0.781); Anti-inflammatory (0.730) |
| 138 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 139 | Lipid metabolism regulator (0.903); Hypolipemic (0.848); Antineoplastic (0.805); Antifungal (0.782) |
| 140 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 141 | Restenosis treatment (0.827); Sclerosant (0.738); Neurodegenerative diseases treatment (0.722) |
| 142 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 143 | Antineoplastic (0.880); Antiviral (Arbovirus) (0.829); Apoptosis agonist (0.804) Hypolipemic (0.794); Antiprotozoal (Coccidial) (0.684); Antiviral (Picornavirus) (0.599) |
| 144 | Antineoplastic (0.885); Antiviral (Arbovirus) (0.814); Apoptosis agonist (0.800) Hypolipemic (0.794); Antiprotozoal (Coccidial) (0.621); Antiviral (Picornavirus) (0.598) |
| 145 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
| 146 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
| 147 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
* Only activities with Pa > 0.5 are shown.
According to PASS data, among methyl-branched FA (119–133), of particular interest is FA (127). A rare feature of this acid that has been shown to be anti-psoriatic and anti-eczematic under the general concept of dermatologic activity with a high certainty of over 90%. The 3D graph of this methyl-branched FA (127) is shown in Figure 35.
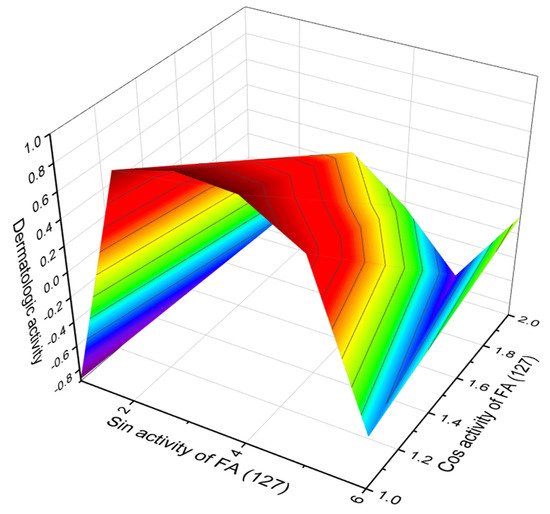
Figure 35. 3D graph showing the predicted and calculated dermatologic activity of methyl-branched FA (127). This acid is incorporated into the cyclic depsipeptide halipeptin A, which is found in the marine sponge Haliclona sp.
(4E,6E)-2,3-Dihydroxy-2,6,8-trimethyldeca-4,6-dienoic acid (139) has been found in the depsipeptides papuamide A–D, which are produced by the sponge Theonella [262,263]. HIV-inhibitory depsipeptides, mirabamides A–D, contain (2R,3R,4R)-3-hydroxy-2,4,6-trimethyloctanoic acid (140) and were extracted from Siliquariaspongia mirabilis, while the cyclic depsipeptide neamphamide D also contains this FA and was found in the Australian marine sponge Neamphius huxleyi [266].
It is known that depsipeptides called didemnins, which are cytotoxins and immunosuppressive agents, were first isolated over 40 years ago from the Caribbean tunicate Trididemnum solidum, and contain (2S,4S)-4-hydroxy-2,5-dimethyl-3-oxohexanoic acid (141) [267]. However, recent data indicate that didemnins do not synthesize tunicate, but rather the symbiotic bacteria Tistrella mobilis [268]. These the symbiotic bacteria of the genus Tistrella have been found in marine sponges and appear to synthesize depsipeptides like didemnins [269].
Cytotoxic undecapeptides, theopapuamides and celebesides A–C from the sponge Theonella swinhoei, showed anticancer activity against HCT–116 cells (colon cancer) [270]. (2R,3R)–3–Hydroxy–2,4,6–trimethyloctanoic acid (142) was present in the undecapeptides theopapuamide A–D, (2E,4E,7S,8R,9S,10R)–7,9–dihydroxy–8,10-dimethyltrideca–2,4–dienoic acid (143) was isolated from celebeside A and C, and (2E,4E,7S,8R,9S,10R)–7,9–dihydroxy–8,10–dimethyldodeca–2,4–dienoic acid (144) was found in celebeside B [264]. Cytotoxic cyclic peptides, aciculitins A–C, were found in the active lipid fraction of the lithistid sponge Aciculites orientalis. Aciculitin A, containing FA (145) and FA (146), was present in aciculitin B, and aciculitin C contains FA (147) [271].
According to PASS data, among the group of fatty acids (134–147), glycosidic FA (145, 146 and 147) are of the greatest interest, which demonstrate anti-infective and antineoplastic activities with a high degree of confidence, more than 93%. Figure 36 demonstrates the 3D graph of the activities of these acids.
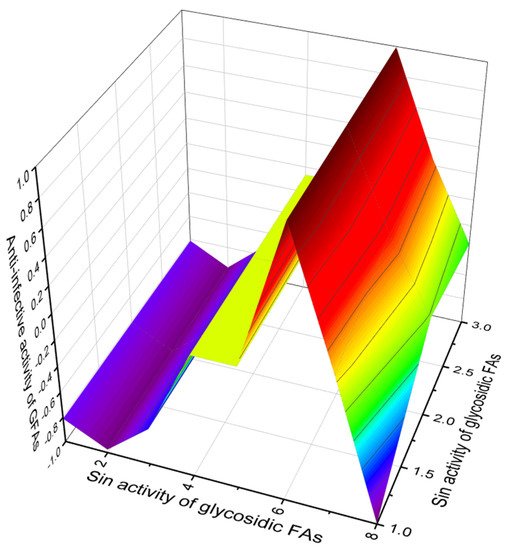
Figure 36. 3D graph showing the predicted and calculated anti-infective and antineoplastic activities of glycosidic FA (145, 146 and 147). These acids are incorporated into cyclic peptides called aciculitins A–C and are produced by the lithistid sponge Aciculites orientalis.
A potent cytotoxin, psymberin, also known as irciniastatin A, is found in the sponge Psammocinia sp. with (2S)-2-hydroxy-3-methoxy-5-methylhex-5-enoic acid (148, for structure see Figure 37, and predicted activity is shown in Table 14) [272,273,274,275] and the keto analogue irciniastatin B was isolated from Ircinia ramosa (Borneo) and contains 2-hydroxy-3-methoxy-5-methylhex-5-enoic acid (149) [276,277].
Figure 37. Branched, saturated, and unsaturated FA derived from sponge lipopeptides.
Table 14. Predicted biological activity of FA derived from peptides of marine sponges.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 148 | Antineoplastic (0.752); Lipid metabolism regulator (0.715); Antiviral (Arbovirus) (0.642) |
| 149 | Antineoplastic (0.752); Lipid metabolism regulator (0.715); Antiviral (Arbovirus) (0.642) |
| 150 | Cell adhesion molecule inhibitor (0.883); Lipid metabolism regulator (0.801); Apoptosis agonist (0.752) |
| 151 | Cell adhesion molecule inhibitor (0.866); Hypolipemic (0.816); Antineoplastic (0.780) |
| 152 | Lipid metabolism regulator (0.931); Antineoplastic (0.826); Apoptosis agonist (0.634) |
| 153 | Lipid metabolism regulator (0.931); Antineoplastic (0.826); Apoptosis agonist (0.634) |
| 154 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) |
| 156 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) |
| 157 | Antineoplastic (0.779); Acute neurologic disorders treatment (0.681); Antiviral (Arbovirus) (0.680) |
| 158 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 159 | Sclerosant (0.834); Antineoplastic (0.799); Acute neurologic disorders treatment (0.725) |
| 160 | Sclerosant (0.835); Antineoplastic (0.795); Acute neurologic disorders treatment (0.731) |
| 161 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 162 | Sclerosant (0.835); Antineoplastic (0.781); Acute neurologic disorders treatment (0.764) |
| 163 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 164 | Antineoplastic (0.881); Hypolipemic (0.797); Antifungal (0.793); Antimitotic (0.787) |
* Only activities with Pa > 0.5 are shown.
Marine sponges belonging to the Jaspidae family produce related bioactive lipopeptides, bengamides [278,279,280,281,282]. Thus, bengamides AE, G, H, J, L, M, O, Y, and Z contain (2R,3R,4S,5R,E)-3,4,5-trihydroxy-2-methoxy-8-methylnon-6-enoic acid (150), bengamides E’ and F’ contain (2R,3R,4S,5R,E)-3,4,5-trihydroxy-2-methoxy-8-methyldec-6-enoic acid (151), bengamides P and Q contain (2R,3R,4R,5R,E)-3,4-dihydroxy-2-methoxy-8-methyl-5-(tetradecanoyloxy)non-6-enoic acid (152), and (2R,3R,4R,5R,E)-3,4-dihydroxy-2-methoxy-8-methyl-5-(palmitoyloxy)non-6-enoic acid (153, activity see in Figure 38) was found in bengamide R [278,279,280,281,282,283].
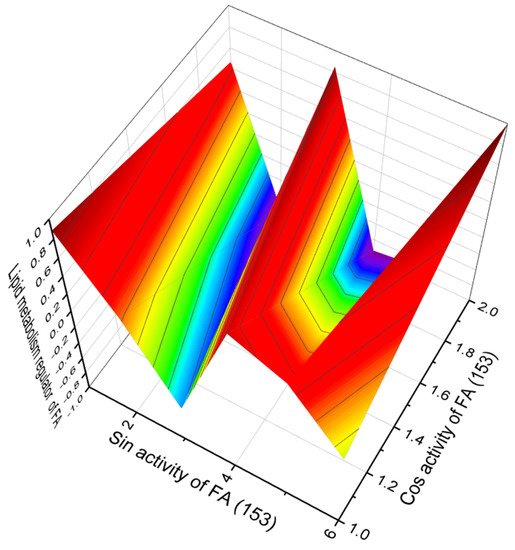
Figure 38. 3D graph showing the predicted and calculated activity of a regulator lipid metabolism of FA (153). Acid 153 has a similar activity since the structures of both metabolites are similar. These acids are found in lipopeptides, bengamides P and Q, and are produced by marine sponges belonging to the Jaspidae family.
(6S,7S,E)-7-Hydroxy-4,4,6,8-tetramethyl-5-oxonon-2-enoic acid (154) was incorporated into a macrocyclic lactam, mirabalin, which was found and isolated from extracts of Siliquariaspongia mirabilis. Mirabalin is known to inhibit the growth of the HCT-116 cell line [284,285,286]. Poecillastrin C and D are isolated from the deep-sea sponge, Japsis serpentine [287,288]. These compounds showed potent cytotoxicity against various tumor cell lines, and both poecillastrins contain (E)-7-hydroxy-4,4,6,8-tetramethyl-5-oxonon-2-enoic acid (153) [287].
An aqueous extract of the marine sponge Chondropsis sp. contains several macrolides called chondropsins [289]. Chondropsin A, B, and D and deoxychondropsin A contain (E)-7-hydroxy-9-methoxy-4,4,6,8,8-pentamethyl-5,9-dioxonon-2-enoic acid (156), and (E)-7-hydroxy-4,4,6,8-tetramethyl-5-oxonon-2-enoic acid (155) was found in chondropsin C [289].
Cytotoxic peptides, theopapuamides A–D, which contain (2R,3R)-3-hydroxy-2,4,6-trimethyloctanoic acid (157) were obtained from Theonella swinhoei sponge extracts [290], while geodiamolide TA was isolated from the marine sponge Hemiasterella minor, which also contained the same FA [291].
The cyclodepsipeptides named homophymines A–E and A1–E1 were obtained from lipid extracts of the marine sponge Homophymia sp. living in the island of Barneo [257,258]. All the members described so far exhibit potent cytotoxic activity. (2R,3R,4R,6R)-3-Hydroxy-2,4,6,8-tetramethylnonanoic acid (158) is found in homophymines B and B1, (2R,3R,4R,6R,8R)-3-hydroxy-2,4,6,8-tetramethyldecanoic acid (159) in homophymines A and A1. Homophymines C and C1 contain (2R,3R,4R,6R)-3-hydroxy-2,4,6-trimethylnonanoic acid (160), homophymines D and D1 contain (2R,3R,4R,6R)-3-hydroxy- 2,4,6,8-tetramethylnonanoic acid (161), and homophymines E and E1 contain (2R,3R,4R,6R)-3-hydroxy-2,4,6,9-tetramethyldecanoic acid (162) [257,258].
The sponge Homophymia lamellosa from the coast of Madagascar yielded cytotoxic cyclic depsipeptides, pipecolidepsins. Both pipecolidepsins A and B contain FA (134), and 3-hydroxy-2,4,6,8-tetramethylnonanoic acid (163) is found in pipecolidepsin C [292].
(2E,4E,7R,8S,9S,10S)-9-Hydroxy-7-methoxy-8,10-dimethyltrideca-2,4-dienoic acid (164) is incorporated into depsipeptide nagahamide A, which demonstrated antibacterial properties and was found in a water–methanol extract of the marine sponge Theonella swinhoei [293].
2.2. Chlorinated Fatty Acids Derived from Sponge Lipopeptides
It is known that chlorinated fatty acids are widely distributed in nature and are part of neutral lipids, phospholipids, and glycolipids, and are also found in natural lipopeptides of marine invertebrates [203]. Polychlorinated peptides from Lamellodysidea herbacea such as dysidin and dysidenin contain (S)-4,4,4-trichloro-3-methylbutanoic acid (165, for structure see Figure 39, and activity is shown in Table 15) [294]. Five dysideaprolines A–F contain FA (166 and 167), and barbaleucamides A and B, which contain (E)-6,6,6-trichloro-3-methoxy-5-methylhex-2-enoic acid (168), were obtained from the Philippines sponge Dysidea sp. (E)-6,6,6-Trichloro-5-methylhex-2-enoic acid, 169) or herbacic acid is the major trichloroleucine metabolite of herbaceamide A in the sponge Dysidea herbacea [294,295] and FA (170) was isolated from chlorinated lipopeptides found in the marine sponge Dysidea sp.
Figure 39. Chlorinated FA derived from sponge lipopeptides.
Table 15. Predicted biological activity of FA from lipopeptides of Dysidea species.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 165 | Antineoplastic (0.841); Preneoplastic conditions treatment (0.689); Antiprotozoal (0.586) |
| 166 | Antineoplastic (0.841); Preneoplastic conditions treatment (0.689); Antiprotozoal (0.586) |
| 167 | Antineoplastic (0.960); Anti-infective (0.613); Acute neurologic disorders treatment (0.572) |
| 168 | Antineoplastic (0.774); Preneoplastic conditions treatment (0.690); Antiprotozoal (0.557) |
| 169 | Antineoplastic (0.850); Antiviral (Arbovirus) (0.765); Acute neurologic disorders treatment (0.574) |
| 170 | Antineoplastic (0.965); Anti-infective (0.628); Acute neurologic disorders treatment (0.589) |
* Only activities with Pa > 0.5 are shown.
Analyzing the PASS data, all chlorine-containing FA (165–170) show the dominant property of anticancer activity with varying degrees of reliability. Strong antitumor activity is characteristic of 165 and 170 acids. Figure 40 demonstrates the 3D graph which shows the predicted and calculated antitumor activity of fatty acid (170).
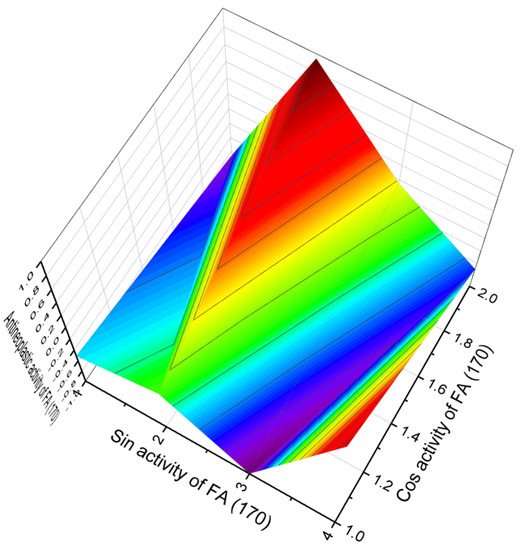
Figure 40. 3D graph showing the predicted and calculated antineoplastic activity of chlorinated FA (170). The figure shows that a single peak (red zone) dominates, which corresponds to the strong antitumor activity of (S)−4,4−dichloro−3−methylbutanoic acid.
2.3. Miscellaneous Fatty Acids Incorporated into Sponge Lipopeptides
A cytotoxic cyclic didepsipeptide named arenastatin A containing (5S,6S,7S,E)-6-hydroxy-5,6-dimethyl-7-(3-phenyloxiran-2-yl)oct-2-enoic acid (171, for structure see Figure 41, and activity is shown in Table 16) was found in a chloroform–methanol extract of the Okinawan marine sponge Dysidea arenaria [296,297,298,299]. This cyclodepsipeptide has an extremely strong cytotoxic activity against KB 3-1 cells (human epidermoid carcinoma cell line) [300,301,302,303].

Figure 41. Miscellaneous FA derived from sponge lipopeptides.
Table 16. Predicted biological activity of FA from sponge lipopeptides.
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 171 | Anti-hypercholesterolemic (0.873); Lipid metabolism regulator (0.713); Atherosclerosis treatment (0.559) |
| 172 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 173 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 174 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 175 | Antiseptic (0.945); Antiinfective (0.900); Preneoplastic conditions treatment (0.818) |
| 176 | Acute neurologic disorders treatment (0.858); Antidiabetic (0.818); Antidiabetic (type 2) (0.645) |
| 177 | Atherosclerosis treatment (0.857); Sweetener (0.635); Restenosis treatment (0.602) |
| 178 | Antihypertensive (0.765); Antidiabetic (0.757); Antithrombotic (0.522) |
| 179 | Antineoplastic (0.855); Transplant rejection treatment (0.591); Autoimmune disorders treatment (0.574) |
| 180 | Antineoplastic (0.667); Angiogenesis stimulant (0.566); Antidiabetic (0.531) |
* Only activities with Pa > 0.5 are shown.
The cytotoxic compounds onnamide A, B, and C were obtained from marine sponge Theonella sp. Onnamide A contains (S)-2-hydroxy-2-((2R,5R,6R)-2-methoxy-5,6-dimethyl-4-methylene-tetrahydro-2H-pyran-2-yl)acetic acid (172), onnamide B contains (S)-2-hydroxy-2-((2S,5R,6R)-2-methoxy-5,6-dimethyl-4-methylenetetrahydro-2H-pyran-2-yl)acetic acid (173), and onnamide C contains (R)-2-hydroxy-2-((2S,5R,6R)-2-methoxy-5,6-dimethyl-4-methylenetetrahydro-2H-pyran-2-yl)acetic acid (174). Onnamide A analogues, 21,22-dihydroxyonnamides A1–A4, containing FA (172), were isolated from an Okinawan collection of Theonella swinhoei [304,305,306]. A cyclic peptide oriamide with 2,5-dihydroxybenzoic acid (175), was detected in the marine sponge Theonella sp. collected in Sodwana Bay [307].
It is known that dysinosin A is an inhibitor of Factor VIIa and thrombin and is produced by the Australian sponge of the family Dysideidae, and contains a sulfated glyceric acid, (R)-2-methoxy-3-(sulfooxy)-propanoic acid (176), as its analogues dysinosins B and C contain this FA (176) [308,309].
The cyclic peptide, scleritodermin A with sodium (S)-(1-carboxy-2-methoxyethyl)-sulfamate (177) inhibited tubulin polymerization and showed significant in vitro cytotoxicity against human tumor cell lines [310] and was isolated from the lithistid sponge Scleritoderma nodosum. The bioactive hexapeptide, keramamide A, from the Okinawan marine sponge Theonella sp. contains (R)-3-formamido-2-hydroxypropanoic acid (178), which was also found in keramamides A, J, K, H, and G [311].
(4R,5S,6E,11E)-5-Hydroxy-4,7,9,11-tetramethyl-12-(oxazol-5-yl)-3-oxododeca-6,11-dienoic acid (179) is incorporated into a cytotoxic lipodepsipeptide named taumycin A, which was obtained from the Madagascar sponge Fascaplysinopsis sp. [256], and the sponge Discodermia kiiensis yielded the cyclic depsipeptides, discokiolide A–C, with (E)-3-hydroxy-2-methyl-3-(2-(4-phenylbut-3-en-2-yl)-oxazol-4-yl)-propanoic acid (180) [312].
According to PASS data, among FA (171–180), tetrahydro-2H-pyran-containing FA (172, 173 and 174) are of the greatest interest, which demonstrate antineoplastic activity with a high degree of certainty, more than 96%. Figure 42 shows the 3D graph of FA (173) activity, and a single peak in the red area corresponds to strong antitumor activity.
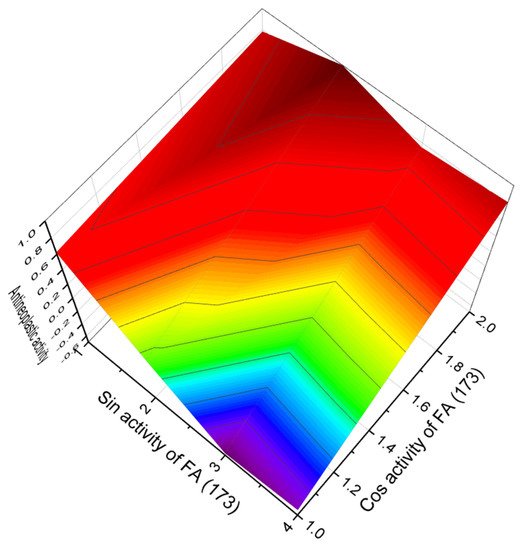
Figure 42. 3D graph showing the predicted and calculated antineoplastic activity of tetrahydro−2H−pyran−containing FA (173). This acid is part of the onnamide B lipopeptide, which exhibits highly cytotoxic activity against the P388 cell line.
Marine sponges, like their freshwater relatives, often contain dense and diverse microbial communities or symbionts, with many microorganisms specific to sponge hosts. Symbiont microorganisms can include bacteria, archaea, and unicellular eukaryotes (fungi and microalgae), and account for up to 40% of the volume of the sponge. These symbionts synthesize a wide variety of organic molecules, including lipopeptides, which can have a profound effect on the biology of the host sponge. To date, there is no definite answer as to who the true producer of certain organic molecules isolated from the body of sponges is. Therefore, when we say that lipopeptides are isolated from sea sponges, this does not mean that these lipopeptides were synthesized by the sponge; they can be synthesized, for example, by fungal endophytes, microalgae, or bacteria.
It is very difficult to characterize the FA composition of lipopeptides in algae and invertebrates, and especially in marine and freshwater sponges. This is since sponges and other invertebrates contain a huge pool of various symbiotic bacteria and fungi. And it is not easy to determine what contribution the symbionts of invertebrates make. It should be noted that most invertebrate lipopeptide FA contain similar fragments of bacterial FA, such as chlorine-containing, oxirane, polymethyl- or phenyl-containing FA. But there are fragments of FA that are not found in bacterial lipopeptides, such as aziridine and tetrahydro-2H-pyran-containing FA.
This entry is adapted from the peer-reviewed paper 10.3390/hydrobiology1030024
This entry is offline, you can click here to edit this entry!
