Ventricular assist devices (VADs) have been considered a reasonable alternative strategy in advanced heart failure (HF), widely used as a bridge to heart transplantation or candidacy. Mechanical unloading supports the function of a failing heart and the perfusion of vital organs through reduced workload placed on the ventricles. In clinical practice, it might initiate a healing response with even myocardial recovery, making heart transplantation and mechanical support no longer required.
- heart failure
- therapy
- VADs
- LVAD
- mechanical unloading
- myocardial recovery
- cardiomyocytes
- mitochondria
- metabolism
1. Mechanical Unloading of Failing Heart
1.1. Ventricular Assist Devices (VADs)
1.2. The Current Status of Left Ventricular Assistance Devices (LVADs) Therapy in Heart Failure
1.3. Bridge to Recovery
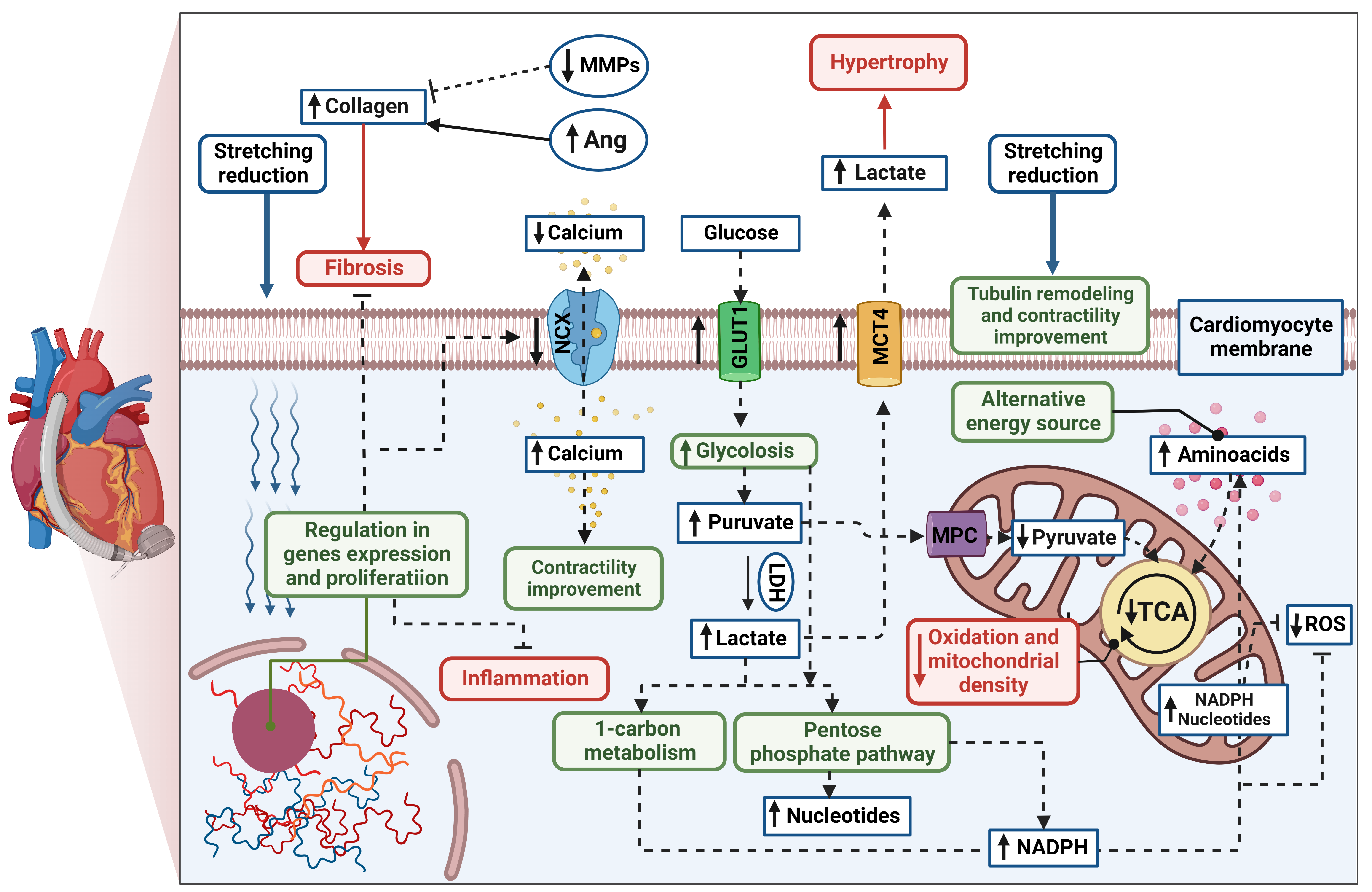
A growing body of evidence has shown that, in respondent patients, LVAD induces structural and functional changes at the cellular, molecular, and whole-heart levels, known as reverse remodelling [6][7][8]. The cellular processes are thought to be more profound and more significant than the changes observed in cardiac function. The potential mechanism of cardiomyocyte remodelling is irreversibly connected to its stretching reduction (Figure 1). Hemodynamic support stimulates karyokinesis and favours the ability to divide, which was confirmed by increasing diploid cardiomyocytes in myocardial samples [9]. An increased number of circulating progenitor cells might correlate to ongoing cardiac recovery; however, their number seems to be transient over time [10][11]. Particular attention has been paid to the specific gene expression in human unloaded hearts, such as expression of profibrotic, contractile, involved in Ca2+ cycling, and proinflammatory proteins [12][13][14]. Reduced level of cytokines was found both in serum and myocardial tissue in patients with improved cardiac function after LVAD implantation compared to the non-responder group [15]. Interestingly, the signal transducer and activator of transcription 3 (STAT3) was responsible for modulating the immune response. Moreover, pre- and post-intervention levels of cytokine were correlated with further LV improvement, suggesting inflammation is an essential factor of LVAD response. It has been highlighted that levels of cytokines in the myocardium, especially tumour necrosis factor (TNF), might predict patients’ recovery [15][16]. The systolic function improvement is thought to be initiated by preserving the abundance of key regulatory proteins (sarcoplasmic reticulum calcium adenosine triphosphatase, SERCA) and a decrease in the Na+/Ca2+ exchanger (NCX) during LVAD therapy [17]. It results in greater calcium uptake and contributes to greater cardiac contractility [18]. Furthermore, mechanical unloading has improved Ca2+ handling through significant tubule remodelling [19]. It has been shown that the density and activity of L-type Ca2+ channels and transverse tubules (t-tubule) have been normalized in the rodent model of mechanical unloading compared to unfavourable outcomes from only HF rats. T-system defects and related-Ca2+ handling aberration are features of heart failure progression and, hence, are thought to be the key to the proper functioning of cardiomyocytes and novel predictors for functional cardiac recovery after mechanical unloading [19][20]. Mechanical support also plays an ambiguous role in extracellular matrix (ECM) remodelling. Lower profibrotic gene expression might contribute to reduced collagen content [21][22]; however, some studies indicated increased fibrosis in heart samples after LVAD support. It can be explained by the decreased breakdown (through decreased activity matrix metalloproteinases activity, MMPs) and increased synthesis of collagen (via increased activity of angiotensin I and II; Ang), which were reported [23][24]. However, ECM turnover is highly related to the aetiology of the injury, RV function, patient’s age, or type of LVAD support, making the influence of mechanical support difficult to determine [25]. Metabolic changes and cellular pathways play a pivotal role in reverse remodelling. It has been reported that effective hemodynamic support induces glycolysis and increases glycolytic metabolites without directing them through the tricarboxylic acid cycle (TCA) [26]. To provide an alternative energy source, the increased level of amino acids was found as a compensatory mechanism. Mitochondrial volume density and mitochondrial DNA (mtDNA), although significantly lower at implantation time compared to the healthy control, have slightly increased during mechanical unloading. Similar results were confirmed in further study, where up-regulated glycolysis initiated activation of protective pathways, such as the pentose phosphate pathway and 1-carbon metabolism in post-LVAD responders [27]. This specific mechanism protects cells against reactive oxygen species (ROS) and increases the synthesis of nucleotides. Furthermore, restoration of the pyruvate–lactate axis was recently highlighted as a predictor of myocardial recovery [28]. Overall, mechanical support causes cascades of reactions in which gene expression, proliferation, apoptosis, fibrosis, immune response, and cardiomyocyte metabolism are modulated. Some of these changes provide prognostic value, and others are the reason why not all LVAD patients achieve myocardial recovery.
1.4. LVAD Limitations
2. Combination of Mechanical Unloading and Pharmacotherapy for Chronic Heart Failure Treatment
2.1. Potential Benefits
2.2. Novel Pharmacotherapies for Cardiomyocyte Regeneration during LVAD Support
2.2.1. Stimulation of Cardiomyocyte Proliferation
2.2.2. Regulators of Cardiac Substrate Metabolism
2.2.3. Mitochondria-Targeted Treatment
Mitochondrial biogenesis, structure, and function have been of special interest with wide clinical testing since their improvement is thought to ameliorate cardiac function directly [89]. The reduction in pathological ROS production is one of the therapeutic targets and is believed to restore energetic balance in cardiomyocytes. To find the optimal antioxidant, supplementation of coenzyme Q (CoQ) was proposed for chronic HF patients in NYHA classification III or IV [90]. CoQ plays a significant role in the mitochondrial electron transport chain as an electron acceptor, thereby contributing to ROS reductions. At week 106 of supplementation, the outcomes were positive and treated patients showed a significantly lower risk of cardiovascular deaths (p = 0.026) and HF hospitalization (p = 0.033) in comparison to the placebo group. These results are in line with a more recent meta-analysis, where reduced mortality and improved exercise capacity were noted in HF patients with CoQ supplementation [91]. Moreover, the CoQ with better mitochondrial bioavailability (MitoQ) was reported to restore mitochondrial respiration and membrane potential in an animal model of heart failure induced by pressure overload [92]. Another rationale for therapeutic use might be decreased endogenous synthesis of CoQ with age [93] and its efficacy in lowering proBNP and improving cardiac systolic function in an elderly population [94]. In turn, the Szeto–Schiller (SS) peptides, especially SS-31, have demonstrated protective properties to cardiolipin, maintaining electron carrying function and ROS utilization [95]. SS-31 safety and toleration have been accepted in two clinical trials, whilst a single infusion in a high dose was beneficial for LV volume [96] but, in the long-term, did not decrease myocardial infarct size [97]. Decreased content of cardiolipin and its mitochondrial decomposition was reported in the myocardium after LVAD support, suggesting that cardiolipin is a potential therapeutic target [98].
To prevent pathological heart remodelling, it is proposed to maintain the nicotinamide adenine dinucleotide (NAD+) pool and NADH (reduced form of NAD+)/NAD+ ratio [99]. An elevated ratio of NADH/NAD+ with cytosolic protein hyperacetylation, including malate–aspartate shuttle proteins and oligomycin-sensitive conferring protein in ATP synthase complex, contributed to the worsening HF in humans and in an animal model of mitochondrial complex-I deficiency. Furthermore, Lee et al. highlighted that elevating the NAD+ level might normalize redox status and improve cardiac function, predicting the high translational potential of the NAD+ precursors. In blood, nicotinamide riboside (NR) was confirmed to successfully increase the level of circulating NAD+ in healthy human voluntaries without serious side effects [100]. Therapeutic opportunities of NR were recently investigated in HF patients undergoing LVAD implantation and showed that oral administration was associated with reduced pro-inflammatory activation [101].
2.2.4. Inhibition of Inflammation
2.2.5. Other Strategies for Cardiac Regeneration
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms23179886
References
- Kadakia, S.; Moore, R.; Ambur, V.; Toyoda, Y. Current status of the implantable LVAD. Gen. Thorac. Cardiovasc. Surg. 2016.
- Iii, W.K.C.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; Koehl, D.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792.
- Task, A.; Members, F.; Mcdonagh, T.A.; United, C.; Gardner, R.S.; Force, T.; United, C.; Baumbach, A.; Kingdom, U.; Bo, M.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 3599–3726.
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086.
- Rose, E.A.; Moskowitz, A.J.; Packer, M.; Sollano, J.A.; Williams, D.L.; Tierney, A.R.; Heitjan, D.F.; Meier, P.; Ascheim, D.D.; Levitan, R.G.; et al. The REMATCH trial: Rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann. Thorac. Surg. 1999, 67, 723–730.
- Birks, E.J.; Drakos, S.G.; Patel, S.R.; Lowes, B.D.; Selzman, C.H.; Starling, R.C.; Trivedi, J.; Slaughter, M.S.; Alturi, P.; Goldstein, D.; et al. Prospective Multicenter Study of Myocardial Recovery Using Left Ventricular Assist Devices (RESTAGE-HF ): Medium-Term and Primary End Point Results. Circulation 2020, 142, 2016–2028.
- Matkovich, S.J.; Van Booven, D.J.; Youker, K.A.; Torre-Amione, G.; Diwan, A.; Eschenbacher, W.H.; Dorn, L.E.; Watson, M.A.; Margulies, K.B.; Dorn, G.W. 2nd Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 2009, 119, 1263–1271.
- Canseco, D.C.; Kimura, W.; Garg, S.; Mukherjee, S.; Bhattacharya, S.; Abdisalaam, S.; Das, S.; Asaithamby, A.; Mammen, P.P.A.; Sadek, H.A. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 2015, 65, 892–900.
- Wohlschlaeger, J.; Schmitz, K.J.; Schmid, C.; Schmid, K.W.; Keul, P.; Takeda, A.; Weis, S.; Levkau, B.; Baba, H.A. Reverse remodeling following insertion of left ventricular assist devices (LVAD): A review of the morphological and molecular changes. Cardiovasc. Res. 2005, 68, 376–386.
- Manginas, A.; Tsiavou, A.; Sfyrakis, P.; Giamouzis, G. Increased Number of Circulating Progenitor Cells After Methods: Results. J. Heart Lung Transplant. 2009, 28, 710–717.
- Ivak, P.; Netuka, I.; Kralova-Lesna, I.; Wohlfahrt, P.; Pitha, J. Changes in circulating stem cells and endothelial progenitor cells over a 12-month period after implantation of a continuous-flow left ventricular assist device. Arch. Med. Sci. 2020, 16, 1440–1443.
- Farris, S.D.; Don, C.; Helterline, D.; Costa, C.; Plummer, T.; Steffes, S.; Mahr, C.; Mokadam, N.A.; Stempien-Otero, A. Cell-Specific Pathways Supporting Persistent Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 344–354.
- Dhar, K.; Moulton, A.M.; Rome, E.; Qiu, F.; Kittrell, J.; Raichlin, E.; Zolty, R.; Um, J.Y.; Moulton, M.J.; Basma, H.; et al. Targeted myocardial gene expression in failing hearts by RNA sequencing. J. Transl. Med. 2016, 14, 327.
- Heerdt, P.M.; Holmes, J.W.; Cai, B.; Barbone, A.; Madigan, J.D.; Reiken, S.; Lee, D.L.; Oz, M.C.; Marks, A.R.; Burkhoff, D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 2000, 102, 2713–2719.
- Diakos, N.A.; Taleb, I.; Kyriakopoulos, C.P.; Shah, K.S.; Javan, H.; Richins, T.J.; Yin, M.Y.; Yen, C.-G.; Dranow, E.; Bonios, M.J.; et al. Circulating and Myocardial Cytokines Predict Cardiac Structural and Functional Improvement in Patients With Heart Failure Undergoing Mechanical Circulatory Support. J. Am. Heart Assoc. 2021, 10, e020238.
- Torre-Amione, G.; Stetson, S.J.; Youker, K.A.; Durand, J.B.; Radovancevic, B.; Delgado, R.M.; Frazier, O.H.; Entman, M.L.; Noon, G.P. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support: A potential mechanism for cardiac recovery. Circulation 1999, 100, 1189–1193.
- Chaudhary, K.W.; Rossman, E.I.; Piacentino, V., 3rd; Kenessey, A.; Weber, C.; Gaughan, J.P.; Ojamaa, K.; Klein, I.; Bers, D.M.; Houser, S.R.; et al. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J. Am. Coll. Cardiol. 2004, 44, 837–845.
- Terracciano, C.M.N.; Harding, S.E.; Adamson, D.; Koban, M.; Tansley, P.; Birks, E.J.; Barton, P.J.R.; Yacoub, M.H. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur. Heart J. 2003, 24, 1329–1339.
- Ibrahim, M.; Navaratnarajah, M.; Siedlecka, U.; Rao, C.; Dias, P.; Moshkov, A.V.; Gorelik, J.; Yacoub, M.H.; Terracciano, C.M. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca2+-induced Ca2+ release in a rodent model of heart failure. Eur. J. Heart Fail. 2012, 14, 571–580.
- Seidel, T.; Navankasattusas, S.; Ahmad, A.; Diakos, N.A.; Xu, W.D.; Tristani-Firouzi, M.; Bonios, M.J.; Taleb, I.; Li, D.Y.; Selzman, C.H.; et al. Sheet-Like Remodeling of the Transverse Tubular System in Human Heart Failure Impairs Excitation-Contraction Coupling and Functional Recovery by Mechanical Unloading. Circulation 2017, 135, 1632–1645.
- Thohan, V.; Stetson, S.J.; Nagueh, S.F.; Rivas-Gotz, C.; Koerner, M.M.; Lafuente, J.A.; Loebe, M.; Noon, G.P.; Torre-Amione, G. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: A comparative analysis with pulsatile-type devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2005, 24, 566–575.
- Müller, J.; Wallukat, G.; Weng, Y.G.; Dandel, M.; Spiegelsberger, S.; Semrau, S.; Brandes, K.; Theodoridis, V.; Loebe, M.; Meyer, R.; et al. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation 1997, 96, 542–549.
- Li, Y.Y.; Feng, Y.; McTiernan, C.F.; Pei, W.; Moravec, C.S.; Wang, P.; Rosenblum, W.; Kormos, R.L.; Feldman, A.M. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 2001, 104, 1147–1152.
- Klotz, S.; Foronjy, R.F.; Dickstein, M.L.; Gu, A.; Garrelds, I.M.; Danser, A.H.J.; Oz, M.C.; D’Armiento, J.; Burkhoff, D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation 2005, 112, 364–374.
- Miyagawa, S.; Toda, K.; Nakamura, T.; Yoshikawa, Y.; Fukushima, S.; Saito, S.; Yoshioka, D.; Saito, T.; Sawa, Y. Building a bridge to recovery: The pathophysiology of LVAD-induced reverse modeling in heart failure. Surg. Today 2016, 46, 149–154.
- Diakos, N.A.; Navankasattusas, S.; Abel, E.D.; Rutter, J.; McCreath, L.; Ferrin, P.; McKellar, S.H.; Miller, D.V.; Park, S.Y.; Richardson, R.S.; et al. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC. Basic to Transl. Sci. 2016, 1, 432–444.
- Badolia, R.; Ramadurai, D.K.A.; Abel, E.D.; Ferrin, P.; Taleb, I.; Shankar, T.S.; Krokidi, A.T.; Navankasattusas, S.; McKellar, S.H.; Yin, M.; et al. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation 2020, 142, 259–274. https://doi.org/10.1161/CIRCULATIONAHA.119.044452.
- Cluntun, A.A.; Badolia, R.; Lettlova, S.; Mckellar, S.H.; Rutter, J.; Drakos, S.G. Article The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure Article The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021, 1–20. https://doi.org/10.1016/j.cmet.2020.12.003.
- Aissaoui, N.; Jouan, J.; Gourjault, M.; Diebold, B.; Ortuno, S.; Hamdan, A.; Latremouille, C.; Pirracchio, R.; Morshuis, M. Understanding left ventricular assist devices. Blood Purif. 2018, 46, 292–300.
- Zimpfer, D.; Strueber, M.; Aigner, P.; Schmitto, J.D.; Fiane, A.E.; Larbalestier, R.; Tsui, S.; Jansz, P.; Simon, A.; Schueler, S.; et al. Evaluation of the HeartWare ventricular assist device Lavare cycle in a particle image velocimetry model and in clinical practice. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2016, 50, 839–848.
- Mariani, S.; Hanke, J.S.; Dogan, G.; Schmitto, J.D. Out of hospital management of LVAD patients during COVID-19 outbreak. Artif. Organs 2020, 44, 873–876.
- Maybaum, S.; Mancini, D.; Xydas, S.; Starling, R.C.; Aaronson, K.; Pagani, F.D.; Miller, L.W.; Margulies, K.; McRee, S.; Frazier, O.H.; et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD working group. Circulation 2007, 115, 2497–2505.
- Antonides, C.F.J.; Schoenrath, F.; De By, T.M.M.H.; Muslem, R.; Yalcin, Y.C.; Netuka, I.; Gummert, J.; Potapov, E.V.; Meyns, B.; Özbaran, M.; et al. Outcomes of patients after successful left ventricular assist device explantation: A EUROMACS study. ESC Heart Fail. 2020.
- Yacoub, M.H. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur. Heart J. 2001, 22, 534–540.
- Hon, J.K.F.; Yacoub, M.H. Bridge to recovery with the use of left ventricular assist device and clenbuterol. Ann. Thorac. Surg. 2003, 75, S36–S41.
- Bowles, C.T.; Ph, D.; Burke, M.; Path, F.R.C.; Banner, N.R.; Khaghani, A. Left Ventricular Assist Device and Drug Therapy for the Reversal of Heart Failure. N. Engl. J. Med. 2006, 1873–1884.
- Groenning, B.A.; Nilsson, J.C.; Sondergaard, L.; Fritz-hansen, T.; Larsson, H.B.W.; Dms, C.; Hildebrandt, P.R.; Dms, C. Antiremodeling Effects on the Left Ventricle During Beta-BlockadeWith Metoprolol in the Treatment of Chronic Heart Failure. Clin. Trial 2000, 36, 2072–2080.
- Greenberg, B.; Quinones, M.A.; Koilpillai, C.; Limacher, M.; Shindler, D.; Benedict, C.; Shelton, B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation 1995, 91, 2573–2581.
- Wong, M.; Staszewsky, L.; Latini, R.; Barlera, S.; Volpi, A.; Chiang, Y.; Benza, R.L.; Gottlieb, S.O.; Kleemann, T.D.; Rosconi, F.; et al. Valsartan Benefits Left Ventricular Structure and Function in Heart Failure: Val-HeFT Echocardiographic Study. Clin. Trial 2002, 40, 970–975.
- Mancini, D.M.; Beniaminovitz, A.; Levin, H.; Catanese, K.; Flannery, M.; DiTullio, M.; Savin, S.; Cordisco, M.E.; Rose, E.; Oz, M. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation 1998, 98, 2383–2389.
- Farrar, D.J.; Holman, W.R.; McBride, L.R.; Kormos, R.L.; Icenogle, T.B.; Hendry, P.J.; Moore, C.H.; Loisance, D.Y.; El-Banayosy, A.; Frazier, H. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2002, 21, 516–521.
- Birks, E.J.; George, R.S.; Hedger, M.; Bahrami, T.; Wilton, P.; Bowles, C.T.; Webb, C.; Bougard, R.; Amrani, M.; Yacoub, M.H.; et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation 2011, 123, 381–390.
- Grupper, A.; Zhao, Y.M.; Sajgalik, P.; Joyce, L.D.; Park, S.J.; Pereira, N.L.; Stulak, J.M.; Burnett, J.C.; Edwards, B.S.; Daly, R.C.; et al. Effect of Neurohormonal Blockade Drug Therapy on Outcomes and Left Ventricular Function and Structure After Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2016, 117, 1765–1770.
- Haider, L.; Hugon-Vallet, E.; Constantin, J.P.; Riad, Z.; Sebbag, L.; Mewton, N. ARNI Pre-Operative Use and Vasoplegic Syndrome in Patients Undergoing Heart Transplantation or Left Ventricular Assist Device Surgery. Med. Sci. 2021, 10, 2.
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E. Angiotensin- Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548.
- Navaratnarajah, M.; Ibrahim, M.; Siedlecka, U.; van Doorn, C.; Shah, A.; Gandhi, A.; Dias, P.; Sarathchandra, P.; Yacoub, M.H.; Terracciano, C.M. Influence of ivabradine on reverse remodelling during mechanical unloading. Cardiovasc. Res. 2013, 97, 230–239.
- Fang, Y.; Debunne, M.; Vercauteren, M.; Brakenhielm, E.; Richard, V.; Lallemand, F.; Henry, J.P.; Mulder, P.; Thuillez, C. Heart rate reduction induced by the if current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J. Cardiovasc. Pharmacol. 2012, 59, 260–267
- Maczewski, M.; Mackiewicz, U. Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc. Res. 2008, 79, 42–51.
- Margulies, K.B.; Matiwala, S.; Cornejo, C.; Olsen, H.; Craven, W.A.; Bednarik, D. Mixed messages: Transcription patterns in failing and recovering human myocardium. Circ. Res. 2005, 96, 592–599.
- Ambardekar, A.V.; Dorosz, J.L.; Cleveland, J.C.; Lindenfeld, J.; Buttrick, P.M. Longitudinal left ventricular structural and functional imaging during full support with continuous-flow ventricular assist devices: A retrospective, preliminary analysis. J. Heart Lung Transplant. 2012, 31, 1311–1313
- Drakos, S.G.; Kfoury, A.G.; Hammond, E.H.; Reid, B.B.; Revelo, M.P.; Rasmusson, B.Y.; Whitehead, K.J.; Salama, M.E.; Selzman, C.H.; Stehlik, J.; et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J. Am. Coll. Cardiol. 2010, 56, 382–391.
- Tran, H.A.; Silva Enciso, J.; Adler, E.D. Often talked about, seldom seen: Promoting myocardial recovery with ventricular assist device. J. Am. Coll. Cardiol. 2014, 64, 1613–1614.
- Michler, R.E. The current status of stem cell therapy in ischemic heart disease. J. Card. Surg. 2018, 33, 520–531.
- Liu, C.-B.; Huang, H.; Sun, P.; Ma, S.-Z.; Liu, A.-H.; Xue, J.; Fu, J.-H.; Liang, Y.-Q.; Liu, B.; Wu, D.-Y.; et al. Human UmbilicalCord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016, 5, 1004–1013.
- Evers, K.S.; Dawoud, F.; George, R.T.; Lima, J.A.C.; Lardo, A.C. CT for Evaluation of Myocardial Cell Therapy in Heart Failure. JCMG 2011, 4, 1284–1293.
- Schuleri, K.H.; Amado, L.C.; Boyle, A.J.; Centola, M.; Saliaris, A.P.; Gutman, M.R.; Hatzistergos, K.E.; Oskouei, B.N.; Zimmet, J.M.; Young, R.G.; et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2002-11.
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in PatientsWith Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal. Circ. Res. 2017, 121, 1192–1204.
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Egeland, T.; Endresen, K.; Ilebekk, A.; Mangschau, A.; Fjeld, J.G.; et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1199–1209.
- Stempien-Otero, A.; Helterline, D.; Plummer, T.; Farris, S.; Prouse, A.; Polissar, N.; Stanford, D.; Mokadam, N.A. Mechanisms of bone marrow-derived cell therapy in ischemic cardiomyopathy with left ventricular assist device bridge to transplant. J. Am. Coll. Cardiol. 2015, 65, 1424–1434.
- Yau, T.M.; Pagani, F.D.; Mancini, D.M.; Chang, H.L.; Lala, A.; Woo, Y.J.; Acker, M.A.; Selzman, C.H.; Soltesz, E.G.; Kern, J.A.; et al. Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in PatientsWith Advanced Heart Failure: A Randomized Clinical Trial. JAMA 2019, 321, 1176–1186.
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.;Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides,M.K.; Jeffries, N.O.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296.
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724.
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470.
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726.
- Huss, J.M.; Kelly, D.P. Mitochondrial energy metabolism in heart failure: A question of balance. J. Clin. Investig. 2005, 115, 547–555.
- Grynberg, A.; Demaison, L. Fatty acid oxidation in the heart. J. Cardiovasc. Pharmacol. 1996, 28 (Suppl. 1), S11-7.
- Dodd, M.S.; Ball, D.R.; Schroeder, M.A.; Le Page, L.M.; Atherton, H.J.; Heather, L.C.; Seymour, A.-M.; Ashrafian, H.; Watkins, H.; Clarke, K.; et al. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovasc. Res. 2012, 95, 69–76.
- Osorio, J.C.; Stanley, W.C.; Linke, A.; Castellari, M.; Diep, Q.N.; Panchal, A.R.; Hintze, T.H.; Lopaschuk, G.D.; Recchia, F.A. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002, 106, 606–612.
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151.
- Lenneman, A.J.; Birks, E.J. Treatment strategies for myocardial recovery in heart failure. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 287.
- Ashrafian, H.; Horowitz, J.D.; Frenneaux, M.P. Perhexiline. Cardiovasc. Drug Rev. 2007, 25, 76–97.
- Cho, Y.W.; Belej, M.; Aviado, D.M. Pharmacology of a new antianginal drug: Perhexiline. I. Coronary circulation and myocardial metabolism. Chest 1970, 58, 577–581.
- Hudak,W.J.; Lewis, R.E.; Kuhn,W.L. Cardiovascular pharmacology of perhexiline. J. Pharmacol. Exp. Ther. 1970, 173, 371–382.
- Cappola, T.P. Perhexiline: Lessons for heart failure therapeutics. JACC Heart Fail. 2015, 3, 212–213.
- Beadle, R.M.;Williams, L.K.; Kuehl, M.; Bowater, S.; Abozguia, K.; Leyva, F.; Yousef, Z.;Wagenmakers, A.J.M.; Thies, F.; Horowitz, J.; et al. Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy. JACC Heart Fail. 2015, 3, 202–211.
- Shu, H.; Peng, Y.; Hang, W.; Zhou, N.; Wang, D.W. Trimetazidine in Heart Failure. Front. Pharmacol. 2020, 11, 569132.
- Ruixing, Y.; Wenwu, L.; Al-Ghazali, R. Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of ischemia-reperfusion. Transl. Res. 2007, 149, 152–160.
- Zhang, J.; He, X.; Bai, X.; Sun, Y.; Jiang, P.; Wang, X.; Li, W.; Zhang, Y. Protective effect of trimetazidine in radiation-induced cardiac fibrosis in mice. J. Radiat. Res. 2020, 61, 657–665.
- Liu, Y.-C.; Li, L.; Su, Q.; Liu, T.; Tang, Z. Trimetazidine pretreatment inhibits myocardial apoptosis and improves cardiac function in a Swine model of coronary microembolization. Cardiology 2015, 130, 130–136.
- Tuunanen, H.; Engblom, E.; Naum, A.; Någren, K.; Scheinin, M.; Hesse, B.; Juhani Airaksinen, K.E.; Nuutila, P.; Iozzo, P.; Ukkonen, H.; et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 2008, 118, 1250–1258.
- Fragasso, G.; Palloshi, A.; Puccetti, P.; Silipigni, C.; Rossodivita, A.; Pala, M.; Calori, G.; Alfieri, O.; Margonato, A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J. Am. Coll. Cardiol. 2006, 48, 992–998. https://doi.org/10.1016/j.jacc.2006.03.060.
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction : A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 6736, 1–11. https://doi.org/10.1016/S0140-6736(20)31824-9.
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA 2020, 323, 1353–1368. https://doi.org/10.1001/jama.2020.1906.
- Trial, E.O.; Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality ? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Randomized Control. Trial 2018, 41, 356–363. https://doi.org/10.2337/dc17-1096.
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221.
- Okopie´ n, B.; Bułdak, Ł.; Bołdys, A. Expert Review of Clinical Pharmacology Benefits and risks of the treatment with fibrates—A comprehensive summary. Expert Rev. Clin. Pharmacol. 2018, 11, 1099–1112.
- Khuchua, Z.; Glukhov, A.I.; Strauss, A.W.; Javadov, S. Elucidating the beneficial role of ppar agonists in cardiac diseases. Int. J. Mol. Sci. 2018, 19, 1–17. https://doi.org/10.3390/ijms19113464.
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. https://doi.org/10.1038/s41569-021-00569-6.
- Javadov, S.; Kuznetsov, A.V. Mitochondria: The cell powerhouse and nexus of stress. Front. Physiol. 2013, 4, 207.
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC. Heart Fail. 2014, 2, 641–649.
- Lei, L.; Liu, Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017, 17, 196.
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.O.; Connell, K.A.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29.
- Kalén, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584.
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866.
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050.
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10.
- Gibson, C.M.; Giugliano, R.P.; Kloner, R.A.; Bode, C.; Tendera, M.; Jánosi, A.; Merkely, B.; Godlewski, J.; Halaby, R.; Korjian, S.; et al. EMBRACE STEMI study: A Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur. Heart J. 2016, 37, 1296–1303.
- Heerdt, P.M.; Schlame, M.; Jehle, R.; Barbone, A.; Burkhoff, D.; Blanck, T.J.J. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann. Thorac. Surg. 2002, 73, 1216–1221.
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation 2016, 134, 883–894. https://doi.org/10.1161/CIRCULATIONAHA.116.022495.
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. https://doi.org/10.1371/journal.pone.0186459.
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. https://doi.org/10.1172/JCI138538.
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation. Circ. Res. 2016, 119, 159–176.
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206.
- Testa, M.; Yeh, M.; Lee, P.; Fanelli, R.; Loperfido, F.; Berman, J.W.; LeJemtel, T.H. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996, 28, 964–971.
- Mann, D.L. Past, Present, and the Foreseeable Future Scientific Rationale for Studying. Circ. Res. 2002, 91, 988–999.
- Tabit, C.E.; Coplan, M.J.; Chen, P.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Tumor necrosis factor- levels and non-surgical bleeding in continuous-flow left ventricular assist devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 107–115.
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.;Willerson, J.T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure. Circulation 2003, 107, 3133–3140.
- Sliwa, K.; Woodiwiss, A.; Kone, V.N.; Candy, G.; Badenhorst, D.; Norton, G.; Zambakides, C.; Peters, F.; Essop, R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: Results of a randomized study. Circulation 2004, 109, 750–755.
- Lecour, S.; Smith, R.M.;Woodward, B.; Opie, L.H.; Rochette, L.; Sack, M.N. Identification of a novel role for sphingolipid signaling in TNF and ischemic preconditioning mediated cardioprotection. J. Mol. Cell. Cardiol. 2002, 34, 509–518.
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. https://doi.org/10.1016/j.amjcard.2013.01.287.
- Van Tassell, B.W.; Abouzaki, N.A.; Oddi Erdle, C.; Carbone, S.; Trankle, C.R.; Melchior, R.D.; Turlington, J.S.; Thurber, C.J.; Christopher, S.; Dixon, D.L.; et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study. J. Cardiovasc. Pharmacol. 2016, 67, 544–551. https://doi.org/10.1097/FJC.0000000000000378.
- Buckley, L.F.; Abbate, A. Interleukin-1 Blockade in Cardiovascular Diseases: From Bench to Bedside. BioDrugs 2018, 32, 111–118. https://doi.org/10.1007/s40259-018-0274-5.
- Elzman, C.R.H.S.; Al, H.E.T. Interleukin-1 Receptor Antagonism as Adjunct Therapy for Heart Failure Patients with Left Ventricular Assist Devices. Clin. Trial 2021, 67, 145–147. https://doi.org/10.1097/MAT.0000000000001347.
- Samuel, T.J.; Rosenberry, R.P.; Lee, S.; Pan, Z. Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. Int. J. Mol. Sci. 2018, 19, 1086.
- del Monte, F.; Harding, S.E.; Schmidt, U.; Matsui, T.; Kang, Z.B.; Dec, G.W.; Gwathmey, J.K.; Rosenzweig, A.; Hajjar, R.J. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999, 100, 2308–2311.
- Zsebo, K.; Yaroshinsky, A.; Rudy, J.J.;Wagner, K.; Greenberg, B.; Jessup, M.; Hajjar, R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: Analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014, 114, 101–108.
- Lyon, A.R.; Babalis, D.; Morley-Smith, A.C.; Hedger, M.; Suarez Barrientos, A.; Foldes, G.; Couch, L.S.; Chowdhury, R.A.; Tzortzis, K.N.; Peters, N.S.; et al. Investigation of the safety and feasibility of AAV1/SERCA2a gene transfer in patients with chronic heart failure supported with a left ventricular assist device-the SERCA-LVAD TRIAL. Gene Ther. 2020, 27, 579–590.
- Chioncel, O.; Collins, S.P.; Butler, J. Istaroxime in acute heart failure: The holy grail is at HORIZON? Eur. J. Heart Fail. 2020, 22, 1694–1697.
- Carubelli, V.; Zhang, Y.; Metra, M.; Lombardi, C.; Felker, G.M.; Filippatos, G.; O’Connor, C.M.; Teerlink, J.R.; Simmons, P.; Segal, R.; et al. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: A randomised, placebo-controlled trial. Eur. J. Heart Fail. 2020, 22, 1684–1693.
- Metra, M.; Chioncel, O.; Cotter, G.; Davison, B.; Filippatos, G.; Mebazaa, A.; Novosadova, M.; Ponikowski, P.; Simmons, P.; Soffer, J.; et al. Safety and Efficacy of Istaroxime for Patients with Acute-Heart-Failure-Related Pre-cardiogenic Shock—A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study (SEISMiC). Eur. J. Heart Fail. 2022, doi:10.1002/ejhf.2629.
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21. https://doi.org/10.3390/ijms21124314.
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. https://doi.org/10.1186/s13293-017-0152-8.
- Iorga, A.; Li, J.; Sharma, S.; Umar, S.; Bopassa, J.C.; Nadadur, R.D.; Centala, A.; Ren, S.; Saito, T.; Toro, L.; et al. Rescue of Pressure Overload-Induced Heart Failure by Estrogen Therapy. J. Am. Heart Assoc. 2016, 5. https://doi.org/10.1161/JAHA.115.002482.
- Lagranha, C.J.; Deschamps, A.; Aponte, A.; Steenbergen, C.; Murphy, E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010, 106, 1681–1691. https://doi.org/10.1161/CIRCRESAHA.109.213645.
- Gardner, J.D.; Murray, D.B.; Voloshenyuk, T.G.; Brower, G.L.; Bradley, J.M.; Janicki, J.S. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H497-504. https://doi.org/10.1152/ajpheart.00336.2009.
- Frump, A.L.; Albrecht, M.; Yakubov, B.; Breuils-Bonnet, S.; Nadeau, V.; Tremblay, E.; Potus, F.; Omura, J.; Cook, T.; Fisher, A.; et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J. Clin. Investig. 2021, 131. https://doi.org/10.1172/JCI129433.
